The electrochemical reduction of carbon dioxide (CO2) has emerged as a frontier in sustainable chemistry, steering a conversation about turning a notorious greenhouse gas into valuable products. Traditionally, the focus has been on perfecting the catalysts used in these reactions, with scientists investing considerable effort into enhancing their efficiency and selectivity. However, recent research has shifted the spotlight towards an often-overlooked aspect: the composition of the electrolyte. This change in focus provides new avenues for innovation in controlling reaction selectivity.
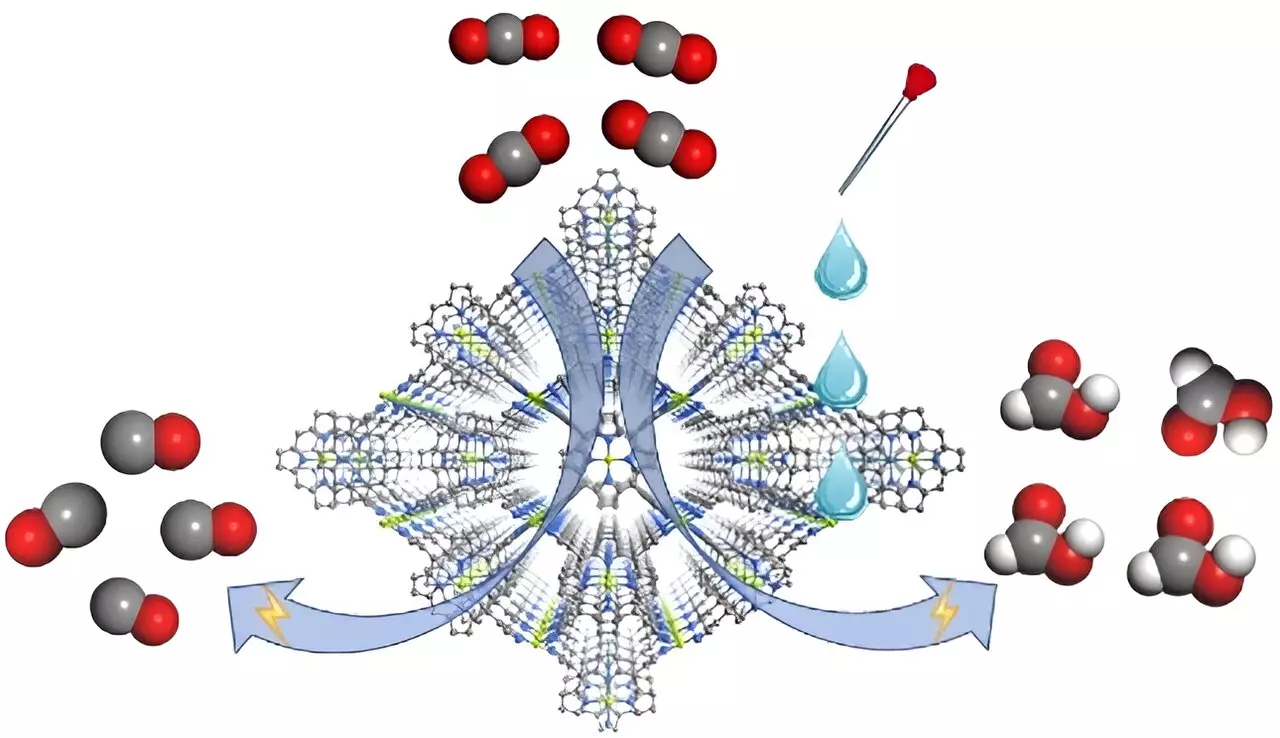
Recent findings published in *Angewandte Chemie International Edition* highlight a groundbreaking investigation into a metal-organic framework (MOF) catalyst. Constructed from a combination of Cu(porphyrin)-derived ligands and Cu(pyrazolate) units, the MOF known as FICN-8 presents a three-dimensional porous architecture that enhances its efficacy as an electrocatalyst. This structural design not only facilitates reactant accessibility but also allows for systematic tuning of the electrolyte composition, a feature that could significantly influence the efficiency of CO2 conversion processes.
The research team, which includes notable scholars like Professors Cao Rong and Zhang Teng, demonstrated that varying the electrolyte could drastically affect the selectivity of the reactions occurring at the metal-organic framework. This discovery marks a pivotal shift in the field—acknowledging that electrolyte composition can offer new strategies for achieving desired reaction products.
The experiments conducted with the FICN-8 catalyst revealed remarkable results in the selective reduction of CO2. In a system using tetrabutylammonium hexafluorophosphate (TBAPF6) dissolved in acetonitrile, the catalyst produced carbon monoxide (CO) with an astonishing selectivity of up to 95%. However, the dynamics changed dramatically with the introduction of protonated solvents like water or trifluoroethanol (TFE). As the proton concentration rose, the primary reduction product shifted from CO to formic acid (HCOOH), showcasing a Faradaic efficiency reaching 48% under optimized conditions.
This transition is not merely an incidental observation but signals the critical role played by proton availability in directing the reaction pathway.
To understand the transformation of CO2 into various products, the researchers employed kinetic isotope effect (KIE) measurements. Results showed a distinct discrepancy in the KIE values between the reactions leading to CO and formic acid production. The significance of these measurements lies in their illustration that protons are closely involved in the formation of formic acid, suggesting a detailed mechanistic pathway that might be manipulated for improved selectivity.
Interestingly, theoretical calculations further elucidated the process, indicating that the adsorption of hydride onto specific nitrogen sites in the porphyrin framework plays a pivotal role in generating formic acid. This information reinforces the conceptual framework that different reaction pathways are influenced by the catalyst’s interaction with the electrolyte composition.
Overall, the exploration of electrolyte composition alongside catalytic design presents a dual approach to optimizing CO2 reduction processes. The collaborative work from the Fujian Institute of Research on the Structure of Matter propels the field towards new catalyst-electrolyte systems aimed at producing high-value products from CO2. This pioneering research not only expands our understanding of electrocatalytic processes but also emphasizes the necessity of integrated strategies in tackling global challenges like carbon emissions.
The study signifies a transformative step in electrochemical CO2 reduction, unlocking potential pathways that could lead to economically sustainable solutions for climate change mitigation. As researchers continue to explore these dimensions, the hope is to develop more efficient technologies that capitalize on CO2’s utility rather than its detriment.