The quest for more efficient energy storage systems has propelled researchers toward the advancement of solid-state battery technology. At the forefront of this domain are lithium and sodium metal anodes, which are pivotal in enhancing the performance of batteries. Recent research from Justus Liebig University Giessen (JLU), in collaboration with teams from the United States and Canada, marks a significant milestone in our understanding of the microstructure of these reactive metals, opening avenues for innovations in battery design and functionality.
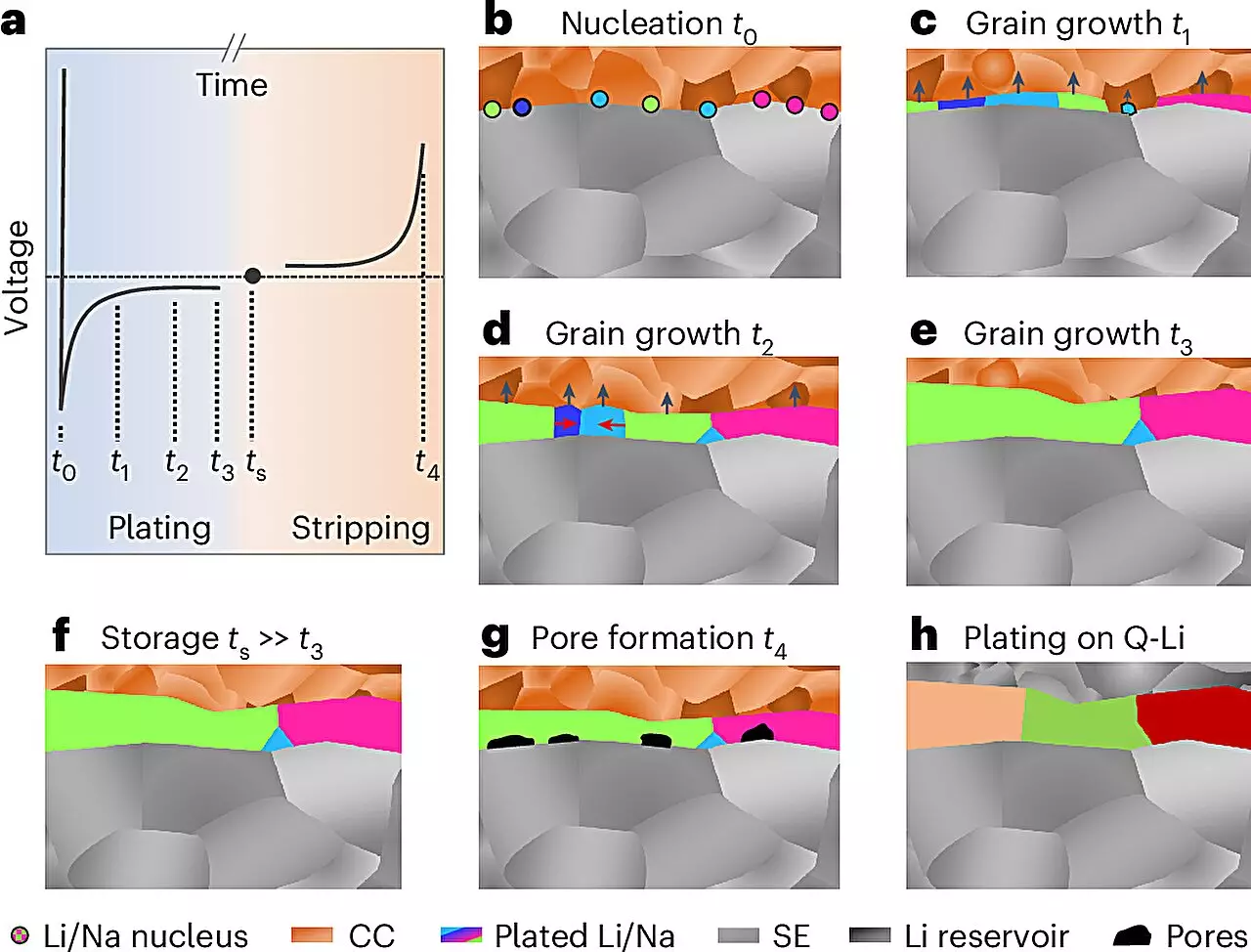
The microstructure of metals, defined as their arrangement and morphology at the nanoscale to microscale, significantly influences their electrochemical characteristics. In contemporary battery technology, particularly for metals like lithium and sodium, understanding this aspect has been complicated by their chemically reactive nature. They form protective reaction layers almost instantaneously when exposed to the environment, obscuring the details of their underlying structures.
Previous research thoroughly explored the microstructures of many metals utilized in various industries, aided by established methods of material science. However, the intricate microstructural dynamics of alkali metals have remained elusive until now. The research team, led by Professor Dr. Jürgen Janek at JLU’s Institute of Physical Chemistry, unveiled a novel method that makes it possible to analyze the microstructure of alkali metals deposited in batteries—a groundbreaking achievement in the domain of materials science.
The methodology harnessed involves a meticulous sequence of preparation and analysis conducted at low temperatures under inert gas conditions, a strategy that detours the complications posed by the reactivity of these alkali metals. The use of electron backscatter diffraction (EBSD) played a crucial role in this process, allowing researchers to visualize and interpret the microstructural features of lithium and sodium layers that were electrochemically deposited.
In particular, the team focused on the formation of these metal layers, achieving thicknesses of nearly 100 micrometers. Their findings revealed surprising grain sizes and provided critical insights into the growth mechanisms of these metals, shedding light on how these materials traverse the transition from deposition to practical application in batteries.
The incorporation of lithium and sodium metal electrodes in solid-state batteries poses significant challenges. One of the most concerning issues is the tendency for these metals to deform during operation, leading to detrimental effects during both charging and discharging cycles. This deformation can result in the formation of pores and dendrites. Dendrites are microscopic, tree-like structures that can puncture through the electrolyte, leading to short circuits—a pronounced safety risk for battery users.
The ideal scenario for solid-state battery development is to restrict the growth of lithium or sodium metal to the initial charging phases, mitigating the handling challenges linked with these volatile materials. Research efforts are ongoing to achieve this goal, and the new insights gained from the JLU-led study provide a solid foundation for further exploration.
Collaboration and Future Directions
The collaborative work between the JLU team and researchers from the University of California, Santa Barbara, and the University of Waterloo has proven invaluable. The seamless integration of expertise in materials science and chemistry enabled the successful imaging of lithium and sodium electrodes—a task that had previously been considered highly difficult. The research has invigorated efforts within established initiatives, such as the POLiS (Post Lithium Energy Storage) excellence cluster, focusing on advancing sodium battery research.
Driven by an ambition to outpace conventional lithium-ion technologies, researchers now possess a deeper appreciation of how microstructural characteristics influence battery performance. As this multidisciplinary collaboration progresses, further breakthroughs are anticipated, further enhancing our understanding of the fundamental properties of alkali metal anodes and propelling the field of solid-state batteries onto a promising trajectory.
The revelation of the microstructural characteristics of lithium and sodium metals represents a monumental step in the evolution of battery technology. With newly developed methodologies and collaborative efforts, the pathway toward high-performance solid-state batteries appears ever more attainable, promising improved safety, longevity, and efficiency in energy storage solutions.