The realm of nuclear material analysis faces continuous challenges, especially when it comes to ensuring the security and regulation of nuclear substances. Recently, researchers from the Department of Energy’s Oak Ridge National Laboratory (ORNL) have made significant strides in this field. By merging two advanced analytic techniques, they have achieved the unprecedented ability to simultaneously detect fluorine and various uranium isotopes within a single particle. This breakthrough is not just a technical marvel; it holds substantial implications for international nuclear oversight and material verification processes.
Published in the esteemed Journal of the American Chemical Society, this study marks a pivotal moment in the field of analytical chemistry. The ability to identify both fluorine and uranium isotopes concurrently offers vital insights into the enrichment processes of uranium, a critical aspect for the International Atomic Energy Agency (IAEA) as they monitor potential nuclear proliferation. Benjamin Manard, the lead investigator of the study, emphasized the significance of speedy isotopic analysis, stating, “Rapid particle analysis for fluorine and uranium isotopic determination is what we’ve enabled.” In doing so, ORNL is pushing the boundaries of how we understand and characterize minute samples that are vital in ensuring safety and compliance in nuclear energy applications.
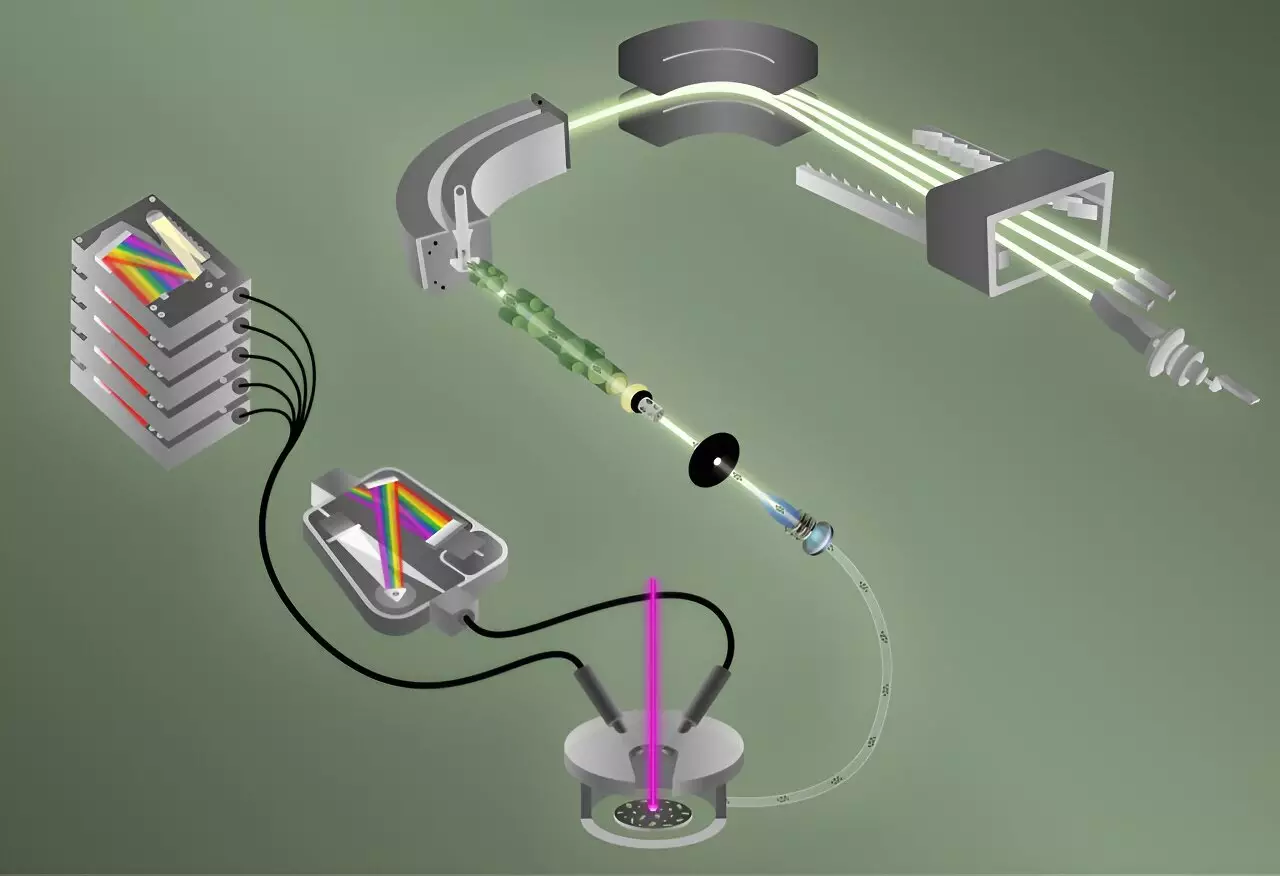
The researchers employed two sophisticated methodologies for this analysis: laser-induced breakdown spectroscopy (LIBS) and laser ablation multicollector inductively coupled plasma mass spectrometry (ICP-MS). Each technique plays a critical role in the overall detection process. LIBS serves as the initial tactic, rapidly recognizing the presence of fluorine with incredible sensitivity. The process involves vaporizing a sample particle—akin to uranyl fluoride—resulting in the formation of a hot plasma. As this plasma emits light as it cools, different elements present can be characterized by their unique spectral signatures, much like a fireworks display, according to Manard.
Following this step, helium gas transports atoms from the plasma into the mass spectrometer, where the ICP-MS method takes over. This process involves using radio-frequency energy to heat the plasma to a staggering 8,000 kelvins, enabling comprehensive analysis of uranium isotopes. This dual approach not only improves accuracy but also expedites the time taken for analysis, allowing the team to analyze 40 particles—each comparable in size to a red blood cell—in under five minutes.
The combined detection of uranium and fluorine within a single particle opens new avenues for monitoring and controlling nuclear materials. A detailed understanding of the ratios between these elements can provide invaluable information regarding the origins, processing, and history of the nuclear materials in question. Brian Ticknor, a co-author of the study, aptly pointed out that this level of detail is invaluable from a nuclear non-proliferation perspective, providing insight into how particular materials have been manipulated or enriched over time.
Interestingly, the applications of this dual-method technique extend far beyond nuclear regulation. The researchers have discussed potential utilization in various fields, including advanced battery technology and environmental science. For instance, by analyzing shark teeth collected from the Georgia Aquarium, they have been able to deduce environmental conditions from the fluorine content, thus illustrating the multifaceted benefits of their novel approach.
A lingering question remains as to why the integration of these two technologies has not been pursued earlier. The compatibility issues arise because while ICP-MS typically detects positively charged ions—a property common to many metals and uranium—it does not easily accommodate elements like fluorine, which are more electronegative and tend to exist as negative ions. Consequently, the researchers at ORNL have paved the way for a new standard in particle analysis by creatively coupling LIBS and ICP-MS, heralding a future where simultaneous isotopic characterization is routinely possible.
Moving forward, the team envisions a broader range of applications for their innovative technique, including distinguishing between different uranium compounds and advancing research into other electronegative elements such as chlorine. Manard expresses a forward-thinking sentiment, indicating a drive to continually enhance the speed and efficiency of particle analysis, aiming for a future where thousands of particles can be processed in just 24 hours.
The work being done at ORNL represents a remarkable convergence of technology and science that promises to redefine nuclear material analysis. Their successful integration of LIBS and ICP-MS is a significant step toward more sophisticated and timely methods of ensuring nuclear safety and compliance, with implications that stretch across numerous scientific disciplines. As these methods evolve, the potential for broadening their applications could benefit various fields, providing crucial insights into both current and historic environmental conditions and processes. This alignment of research priorities with real-world challenges exemplifies the innovative spirit inherent in the scientific community.