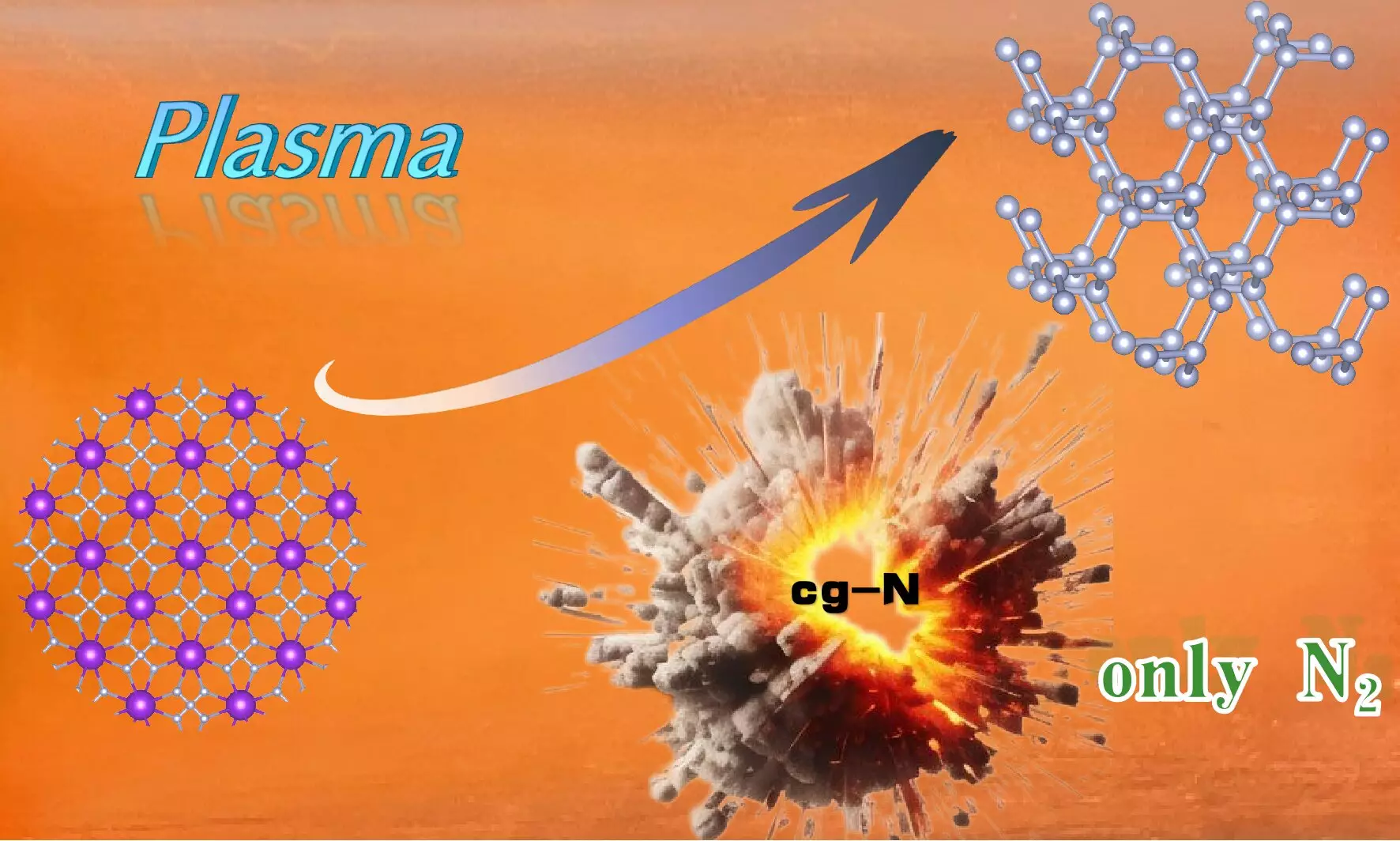
In a pioneering effort to enhance the synthesis of high-energy-density materials, a team of researchers led by Prof. Wang Xianlong at the Hefei Institutes of Physical Science, part of the Chinese Academy of Sciences, has achieved a remarkable milestone. Their groundbreaking work, published in the journal Science Advances, involves the successful synthesis of cubic gauche nitrogen (cg-N) at atmospheric pressure using an innovative technique known as plasma-enhanced chemical vapor deposition (PECVD) on potassium azide (KN3). This advancement not only pushes the boundaries of materials science but also has potential implications for a variety of industries requiring efficient and safe energy storage solutions.
Cubic gauche nitrogen is a novel material characterized by its high-energy density. Composed purely of nitrogen atoms, cg-N is held together by N-N single bonds, mirroring the structural arrangements found in diamonds. As a relatively stable form of nitrogen, it stands out because its decomposition yields only nitrogen gas, making it an environmentally friendly option for high-energy applications. The attention garnered by cg-N arises not just from its innovative structure, but also from its potential uses in energy systems, explosives, and other fields where performance and safety are paramount.
The ability to synthesize cg-N at atmospheric pressure has long been an arduous challenge for researchers. The stability of cg-N has often been compromised under various conditions, especially at low pressures where surface instability can lead to premature decomposition. To tackle this issue, the research group employed first-principles calculations as theoretical frameworks to explore the stability of cg-N under a wide range of conditions. Their studies indicated that stabilizing the surface suspension bonds and enhancing charge transfer could make cg-N viable at temperatures up to 750 K while under atmospheric conditions.
A significant aspect of their innovation lies in the selection of potassium azide (KN3) as a precursor for the synthesis. Recognizing the challenges posed by materials with higher toxicity and explosive potential, the researchers opted for KN3 due to its lower risk profile and its potent electron transfer capabilities. The use of PECVD technology eliminated the reliance on the carbon nanotube-limiting effect, further streamlining the synthesis process.
Thermogravimetric-differential scanning calorimetry (TG-DSC) tests have validated the thermal stability of the synthesized cg-N, demonstrating resilience up to 760 K before a rapid and intense thermal decomposition occurs. This finding is pivotal as it not only confirms the viability of the synthesis method but also highlights the material’s potential in practical applications.
The successful synthesis of cubic gauche nitrogen at atmospheric pressure marks a significant step forward in the development of high-energy-density materials. The methodologies and insights derived from this study could lay the foundation for exploring new materials with similar properties, thereby expanding the arsenal available to researchers and industry professionals alike in the quest for safer and more efficient energy solutions. As the material landscape continues to evolve, the work of Prof. Wang’s team represents an exciting chapter in the ongoing dialogue about energy sustainability and material innovation.