The pursuit of synthesizing complex organic compounds has always been a cornerstone of chemical research, particularly when it involves creating specific isomers essential for biological activity. Recently, a team of chemists at the National University of Singapore (NUS) has taken a significant step forward in addressing one such challenge concerning the selective production of trisubstituted Z-alkenes. Published in the prestigious journal *Nature Synthesis*, their research focuses on a novel iron-catalyzed approach that facilitates the incorporation of two alkyl groups into allenes, which are a type of reactive compound in organic chemistry.
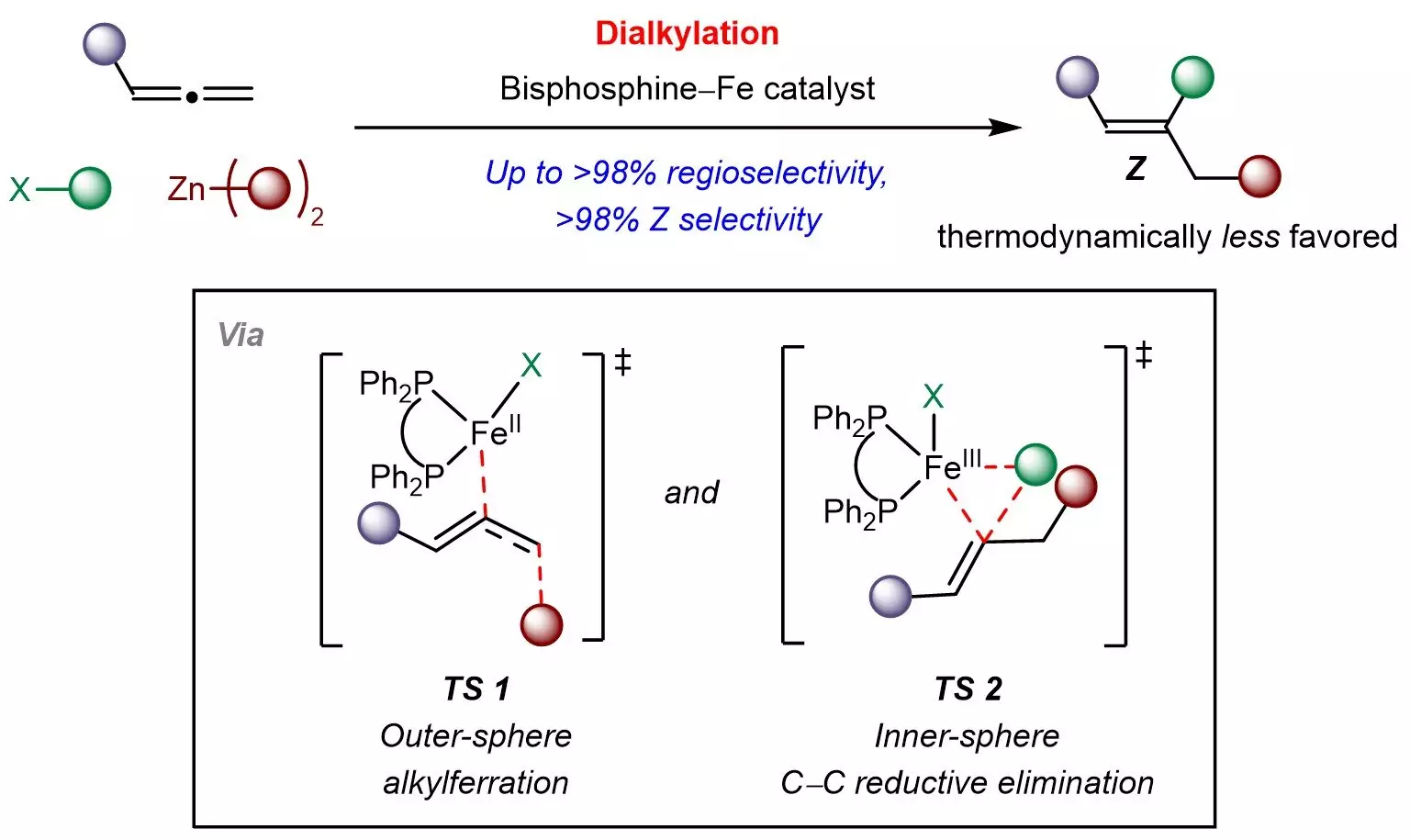
Trisubstituted alkenes play a pivotal role in the synthesis of biologically active molecules, serving as vital intermediates in various stereospecific reactions that create sp3-hybridized scaffolds. However, a significant hurdle in synthesizing these compounds lies in the relative stability of Z-isomers versus their E counterpart. The background knowledge in organic chemistry elucidates that Z-isomers, while often more desirable for specific applications due to their unique chemical properties, are energetically less stable. This instability necessitates a novel catalytic strategy to shift the equilibrium in favor of Z-selectivity.
The research team, led by Associate Professor Koh Ming Joo, addressed this challenge by developing a straightforward methodology that employs an economical bisphosphine-iron catalyst. This catalyst is leveraged to engage with common chemical building blocks such as organohalides and organozinc reagents, allowing for the effectively controlled addition of aliphatic groups to the allene structure. The dual advantage of using iron—an abundant, non-toxic metal—further enhances the sustainability and cost-effectiveness of the entire process, making it an appealing route for further applications.
Moreover, the method not only showcases the versatility in the synthesis of Z-alkenes but also emphasizes the potential for application in synthesizing complex molecules required in pharmaceuticals. One remarkable outcome of this research was the simplified preparation of a glucosylceramide synthase inhibitor, underscoring the importance of Z-configuration in maintaining biological efficacy.
Prof. Koh remarked on the broader implications of their work, highlighting the novel mechanistic insights gained through their studies. Their findings suggest an innovative process involving outer-sphere radical-mediated alkylferration followed by inner-sphere carbon-carbon bond formation, which is critical for the design of kinetically controlled reactions involving allenes. As the research team continues to explore these insights, they are poised to develop additional multicomponent transformations that could transform readily available raw materials into high-value chemical products for diverse industrial sectors.
The NUS chemists have not only bridged a significant gap in the synthesis of trisubstituted Z-alkenes but also laid the groundwork for future research and applications in organic synthesis. By harnessing an iron-catalyzed approach, their work exemplifies how innovative catalysts can both streamline synthetic pathways and promote environmentally sustainable practices in chemistry. The ripple effects of such advancements are likely to benefit not only academic research but also practical applications in drug development and beyond.