As global populations grow and urbanization accelerates, the demand for safe, clean water becomes more critical than ever. Traditional wastewater treatment systems are often ill-equipped to handle the complex array of contaminants present in municipal effluents, particularly pharmaceutical micropollutants. Studies reveal that as many as 4,000 different pharmaceuticals can be found in our water systems, originating from human and veterinary use, often left over and subsequently flushed into drains. Unfortunately, the remnants of these substances can persist in the environment, raising serious concerns over their potential impact on wildlife and ecosystems.
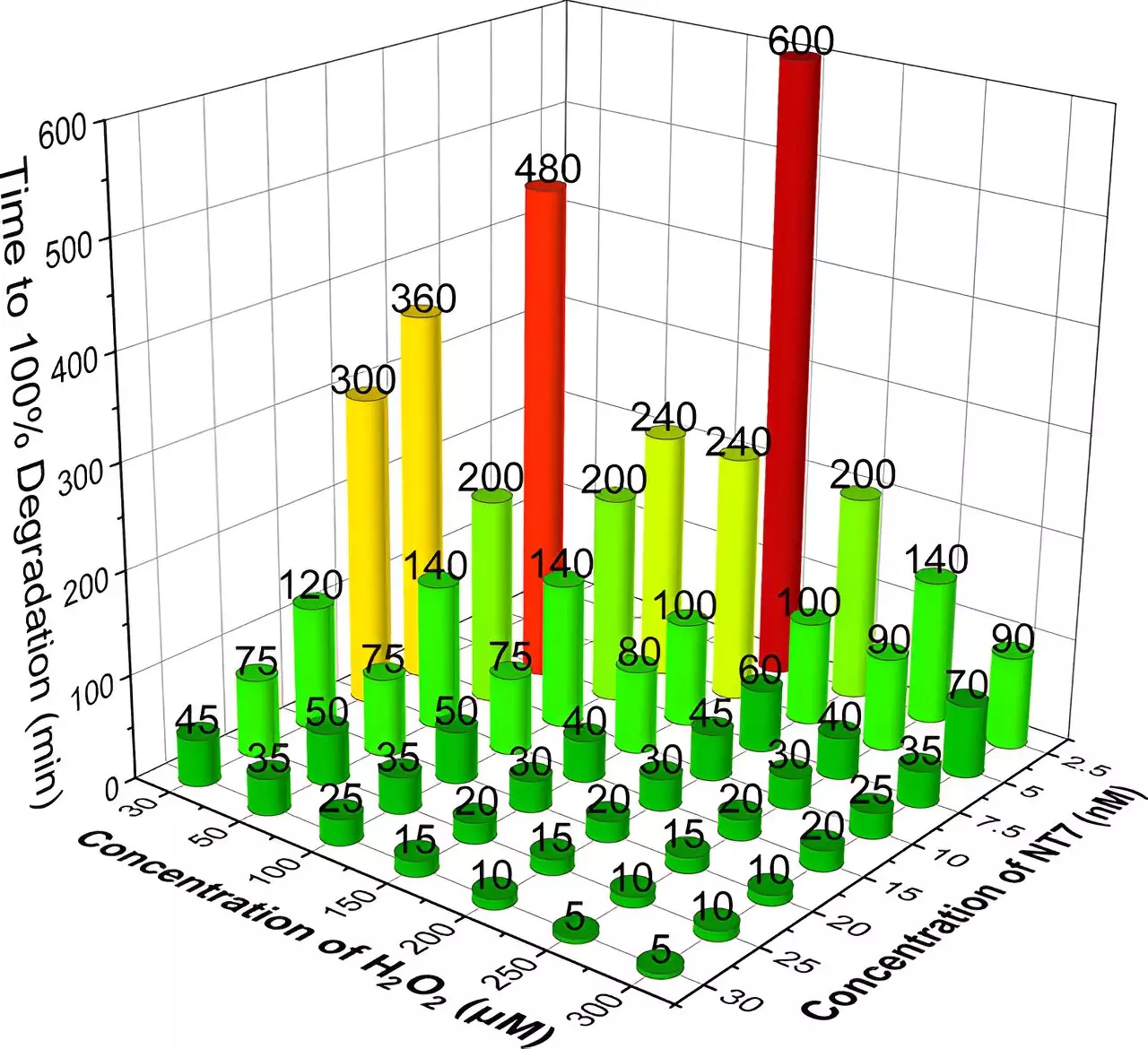
Recent research spearheaded by scientists at Carnegie Mellon University introduces a promising solution to this pressing environmental issue: TAML (Tetramethylammonium-Like) catalysts paired with hydrogen peroxide (H2O2). This innovative approach displays remarkable efficiency in breaking down several high-priority drugs—including antibiotics, synthetic estrogens, and anti-inflammatory medications—demonstrating the potential to transform water purification processes.
The research was published in the journal *ACS Sustainable Chemistry and Engineering*, highlighting the capabilities of what researchers term “NewTAML,” a next-generation catalyst contributing to efficient degradation of pharmaceutical pollutants. Terry Collins, the Teresa Heinz Professor of Green Chemistry at Carnegie Mellon, elaborated on how these catalysts operate at ultra-low concentrations, making the entire purification process not only effective but also cost-efficient and environmentally sustainable.
Understanding TAML Technology
TAML catalysts are remarkable bioinspired compounds; they mimic peroxidase enzymes found in nature. This unique design allows them to activate hydrogen peroxide at extremely diluted concentrations, making it possible to meticulously remove contaminants from various water sources without the need for high levels of chemicals or extensive infrastructure.
The new class of TAML catalysts takes the efficacy observed in previous iterations and amplifies its performance, showcasing unprecedented results in laboratory settings. A chemistry doctoral student, Xiaowei Ma, illustrated the driver of this efficiency: a minuscule amount of NewTAML combined with a small quantity of hydrogen peroxide managed to effectively degrade six high-concern pharmaceuticals within mere hours. In fact, after just six hours under optimal conditions, five of the drugs could not be detected in the tested samples, while the remaining drug saw a degradation rate of up to 95.4%. Such efficacy is notable, especially when considering the high concentrations of drugs initially used in these experiments, significantly exceeding levels typically found in contaminated waters.
Impacts on Wildlife and Ecosystems
One of the most alarming aspects of pharmaceutical contamination in waterways is the potential effects on wildlife. Chronic exposure to micropollutants can lead to altered behavior, reproductive health issues, and even population declines among various species. The TAML/peroxide methodology not only exhibits remarkable degradation rates but also presents a solution that can be readily adopted in various environmental settings, targeting both urban wastewater and natural ecosystems.
The ease of application of this new treatment method is a significant advantage. Unlike other advanced treatment methods, such as ozonation or activated carbon sorption—which often entail complicated processes and high costs—TAML catalysts primarily require the mixing of solutions, streamlining the implementation process. According to Collins, this simplicity opens doors for widespread application in regions lacking advanced wastewater infrastructure.
Looking ahead, the Carnegie Mellon team aims to conduct field tests to further validate the efficacy of the TAML/peroxide technology in real-world settings. They have already begun collaborating with Sudoc, a startup that aims to commercialize these solutions, recently raising $20 million from various investors to accelerate the introduction of TAML-based systems, particularly in the European water treatment market.
The integration of TAML catalysts into wastewater treatment could signal a paradigm shift in how we approach water purification challenges. With their unique properties offering effective, safe, and affordable remediation options, they hold the potential to not only address pharmaceutical contamination but also pave the way for solutions to other pressing environmental issues.
In a world where water is an increasingly precious resource, the development and implementation of innovative purification technologies are essential. This ongoing research by the Carnegie Mellon team sets a precedent for future advancements in clean water technology, underpinning the integration of green chemistry in sustainable solutions for environmental health.