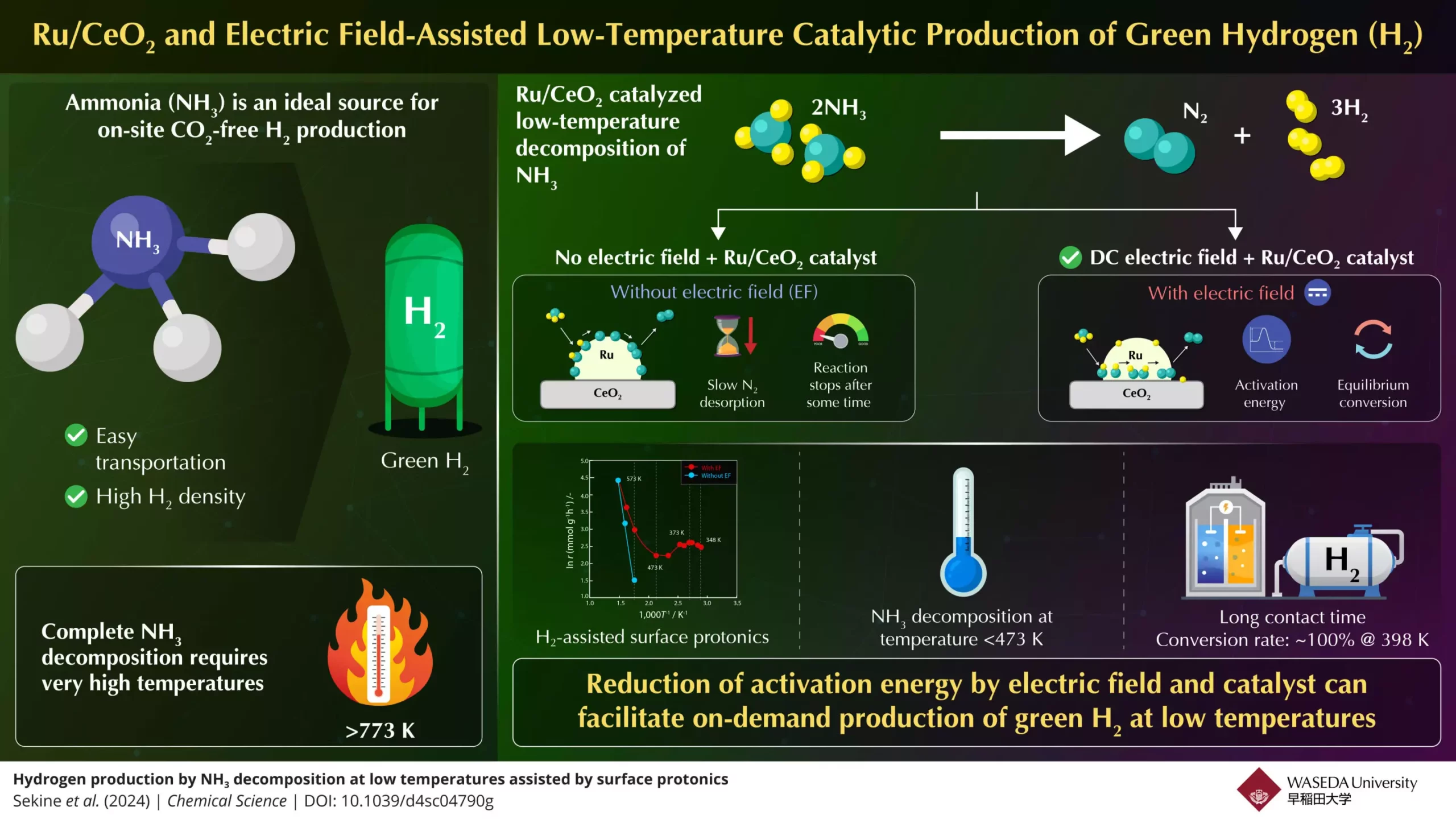
As the global push towards sustainable energy sources intensifies, hydrogen gas emerges as a frontrunner for a greener future. Renowned for its high energy density and environmentally friendly profile—producing only water when burned—hydrogen offers a sustainable solution to today’s pressing energy challenges. Despite its abundance in the universe, primarily as part of chemical compounds like ammonia and metal hydrides, hydrogen’s pathway to becoming a predominant energy carrier has proven complex. Among various hydrogen carriers, ammonia (NH3) has garnered significant attention due to its high hydrogen content and the relative ease of its liquefaction and transport. However, the existing methods for extracting hydrogen from ammonia require unavoidably high temperatures, which pose significant barriers to its practical use.
The efficiency of using ammonia as a hydrogen source is severely compromised by the need for high-temperature processes, typically exceeding 773 K, to decompose it effectively. This requirement limits ammonia’s utility in real-world applications, particularly in fuel cells and internal combustion engines, which demand efficient hydrogen generation at lower temperatures. Thus, developing methods to streamline ammonia’s decomposition process under milder conditions is crucial for facilitating its role in sustainable energy systems.
In a significant breakthrough, Professor Yasushi Sekine and his team from Waseda University, in collaboration with experts from Yanmar Holdings, embarked on pioneering research aimed at overcoming these thermal barriers. The group’s findings, published in the journal Chemical Science, reveal a novel approach that integrates electric field assistance with catalysis to dramatically reduce operating temperatures for ammonia conversion.
The research team discovered that the desorption of nitrogen from the catalyst surface is the slowest step in the ammonia decomposition process when conducted at lower temperatures. To remedy this, they applied a DC electric field to enhance the catalytic activity of a Ru/CeO2 catalyst, which is known for being both effective and readily producible. This innovative modification not only facilitated proton conduction at the catalyst’s surface but also significantly lowered the required activation energy for the reaction. Consequently, this advancement enabled ammonia to be decomposed effectively even below 473 K.
Their experimental setup showcased that, under optimal conditions, 100% ammonia conversion could be achieved at temperatures as low as 398 K. This remarkable accomplishment surpasses traditional equilibrium conversion rates, suggesting a paradigm shift in ammonia utilization as a hydrogen source. The application of an electric field was found to catalyze proton mobility—known as surface protonics—thereby enhancing the reaction kinetics.
This pioneering work opens new avenues for the adoption of hydrogen as a clean alternative fuel. The ability to generate CO2-free hydrogen on demand from ammonia not only addresses the immediate energy needs but also presents a viable solution for integrating hydrogen into the broader energy landscape. The low-temperature operation coupled with an irreversible conversion pathway demonstrates a significant leap towards practical applications, making it easier to harness ammonia’s hydrogen-rich potential.
Moreover, as Professor Sekine notes, this innovative method carries the promise of accelerating the transition to alternative fuels by simplifying the on-demand synthesis of hydrogen. The research indicates not just theoretical advances but real-world applicability, providing a feasible strategy that could lead to more sustainable practices in energy production and consumption.
The ongoing research into ammonia conversion processes exemplifies the commitment to find innovative solutions that address both energy demands and environmental sustainability. By leveraging advancements in catalysis and electric field applications, researchers have paved the way for a practical roadmap to harnessing hydrogen, the universal fuel of the future. As various sectors increasingly recognize the importance of decarbonization in combating climate change, methodologies like this one could very well define the next chapter in the evolution of energy systems. The potential for a cleaner, greener future has never seemed more within reach.