Cholesterol is often demonized in popular discourse, yet it plays an intricate and indispensable role within the architecture of cell membranes. A recent investigation spearheaded by researchers at Rice University, under the guidance of Professor Jason Hafner, has provided fresh insights into the molecular dynamics of cholesterol within biomembranes. This study, featured in the Journal of Physical Chemistry, promises to deepen our understanding of membrane organization and its associated implications for various diseases, particularly cancer.
Cell membranes, composed of an intricate blend of proteins and lipids, are far from simple barriers; they are dynamic structures that play critical roles in cellular communication and function. Cholesterol molecules are embedded within these membranes and are pivotal in modulating membrane fluidity and stability. Despite its significance, deciphering the interactions and positioning of cholesterol within these complex lipid bilayers has posed substantial challenges for scientists.
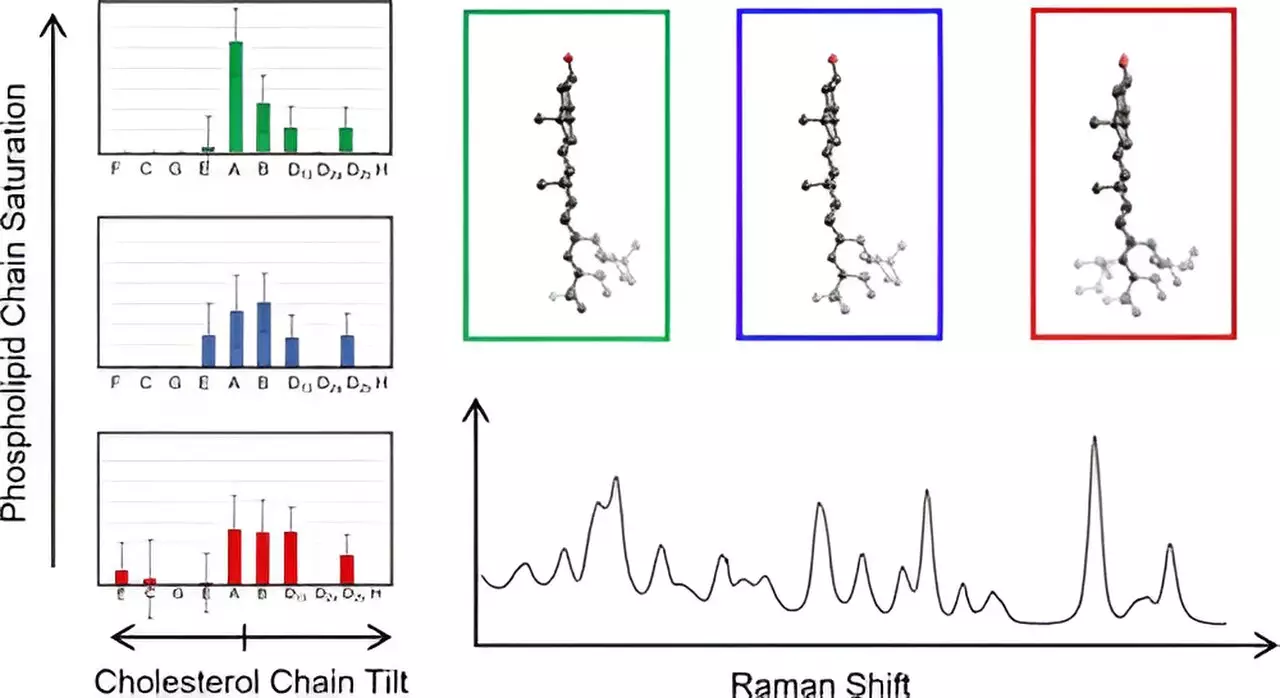
To unravel these complexities, Hafner’s team employed Raman spectroscopy, a powerful analytical technique that uses laser light to probe molecular vibrations. This method allows researchers to collect detailed spectral data that reflect the molecular makeup of lipids and proteins within membranes. By analyzing cholesterol molecules in situ, the researchers could acquire critical information regarding their structural configurations, providing a window into their functional roles. The study utilized density functional theory to compare observed spectral data against theoretical models, establishing a comprehensive understanding of cholesterol’s conformational nuances.
The researchers examined 60 distinct structural configurations of cholesterol, focusing on its unique fused ring structure and accompanying eight-carbon chain. A notable revelation emerged: the researchers discovered a correlation between structural variations and the deviations of the cholesterol chain from the ring plane. This finding highlights the previously underexplored diversity in cholesterol structures, opening new avenues for research into membrane dynamics.
Perhaps most astonishing was the observation that cholesterol molecules sharing similar structural characteristics exhibited identical low-frequency spectral signatures. This uniformity simplifies the analysis process, allowing for a more streamlined mapping of cholesterol dynamics within the membrane environment. Such results could drastically alter our understanding of how membrane cholesterol contributes to cellular functions and, by extension, its role in pathology.
The groundbreaking work by Hafner and his collaborators underscores the importance of cholesterol in maintaining the integrity and functionality of cell membranes. Moving forward, further exploration of cholesterol’s behavior in various membrane contexts could yield vital insights into diseases characterized by membrane dysfunction. Investigating these relationships is essential, particularly in cancer research, where the organization of cell membranes is critical to understanding disease mechanisms. In a broader context, this study sets a precedent for future investigations, underscoring the need for innovative techniques in shedding light on the hidden complexities of biomolecular interactions.