Samarium (Sm), a member of the rare earth metals, has carved a niche for itself in the realm of organic chemistry due to its remarkable capacity for facilitating single-electron transfer reductions through its divalent compounds. Among its various salts, samarium iodide (SmI2) stands out because of its moderate stability and capability to function efficiently at room temperature. This property makes it significantly useful in synthesizing pharmaceuticals and biologically active compounds. However, the application of SmI2 is not without challenges. The conventional methods employed in samarium-based reactions often necessitate stoichiometric quantities of Sm, resulting in a highly resource-intensive and economically burdensome process, particularly because many reactions engage harmful chemicals.
Research has made strides toward minimizing the amount of samarium required by transitioning to catalytic applications rather than stoichiometric ones. Despite these endeavors, many current methodologies still demand harsh reaction conditions and highly reactive reducing agents, which often leads to a usage of samarium at levels of approximately 10–20 percent relative to the starting materials. Given the escalating costs associated with samarium, there is an ever-growing demand for an innovative catalyst system that can operate effectively while employing minimal quantities of this precious metal under milder conditions.
Breakthrough Innovations from Chiba University
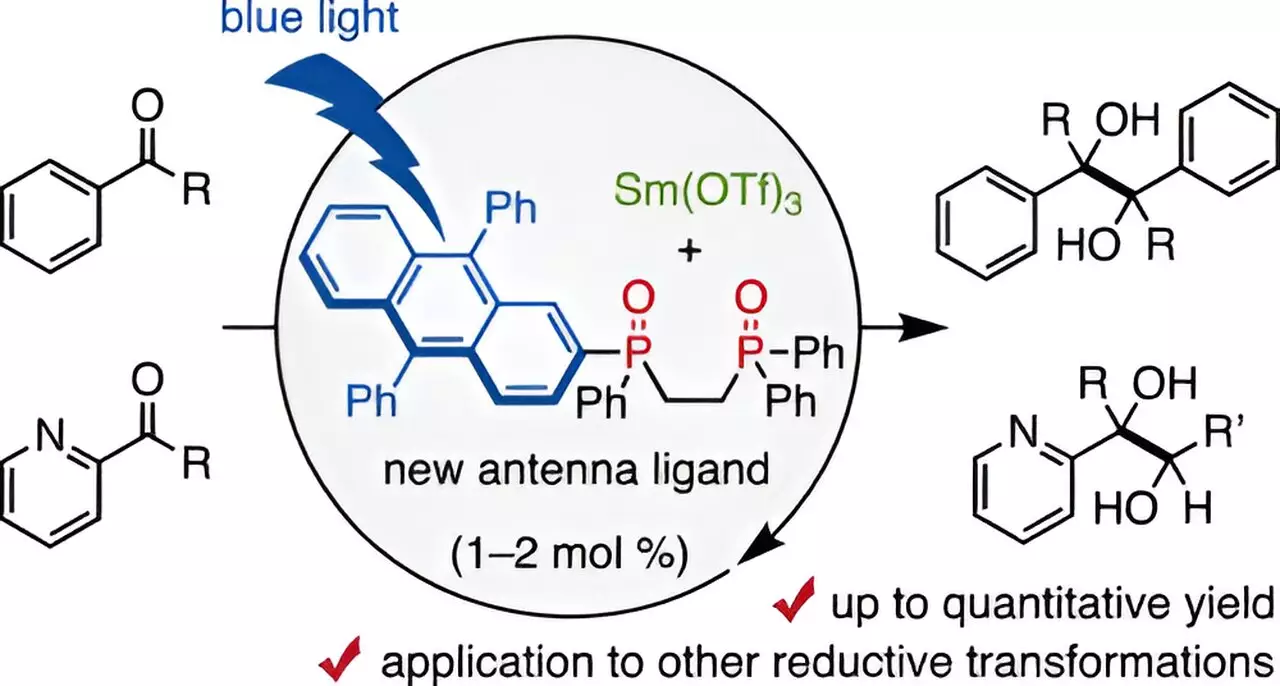
A recent breakthrough from a team led by Assistant Professor Takahito Kuribara at Chiba University illustrates a significant evolutionary leap in this domain. The researchers have pioneered a sophisticated approach utilizing a 9,10-diphenyl anthracene (DPA)-substituted bidentate phosphine oxide ligand, which significantly lowers the required amounts of samarium in catalytic processes. They have creatively fashioned this ligand to serve as a “visible-light antenna,” facilitating reactive transformations catalyzed by samarium through exposure to visible light.
Kuribara elaborates that antenna ligands are known to enhance the excitation of lanthanoid metals, such as samarium, further stating, “By designing a new DPA-substituted bidentate phosphine oxide ligand that employs visible light, we were able to reduce the necessary concentration of Sm to catalytic levels.”
Revolutionary Results and Experimental Validation
Collaborating with colleagues Ayahito Kaneki, Yu Matsuda, and Tetsuhiro Nemoto, Kuribara’s team conducted a series of experiments that showcased how the DPA-1 ligand, when used in conjunction with the Sm catalyst under blue-light irradiation, can drive pinacol coupling reactions of aldehydes and ketones to high yields of up to 98%. Notably, this was achievable with just 1-2 mol% of the samarium catalyst, dramatically less than the standard stoichiometric amounts required in traditional methodologies.
Interestingly, the experiments also uncovered that these reactions could be performed using less intensive organic reducing agents, like amines, instead of the harsher reducing agents that were standard in previous methodologies. The introduction of a small volume of water was found to boost yields; however, excessive water proved to inhibit the reaction, adding another layer of complexity to the process.
To understand the exceptional efficacy of DPA-1 as a ligand, the researchers closely analyzed the photophysical properties and electron transfer mechanisms involved in the radical generation and subsequent reactions. They concluded that DPA-1 acts as a multifunctional ligand that not only coordinates effectively with the Sm catalyst but also selectively absorbs blue light, leading to efficient electron transfer from the antenna to Sm.
Building on this, the team applied their Sm catalyst-DPA-1 system to a variety of pivotal molecular transformation reactions crucial for drug development. This included processes aimed at carbon-carbon bond formation, as well as bond cleavage reactions, which are central to medicinal chemistry. Their innovative method also allowed for the merging of samarium-based reduction with photo-oxidation reactions—all powered by visible light.
The efforts from the Chiba University research team signal a significant advancement in the field of organic chemistry by showcasing a viable system capable of performing Sm-catalyzed reductive transformations using minimal quantities of samarium—reducing the requirement to just 1-2 mol%. This novel methodology not only conserves an expensive resource but also enhances the simplicity and safety of chemical reactions by making use of trivalent samarium, which is more stable and manageable than its divalent counterpart. The insights drawn from this study lay a promising foundation for future explorations and developments in Sm-based catalysis.