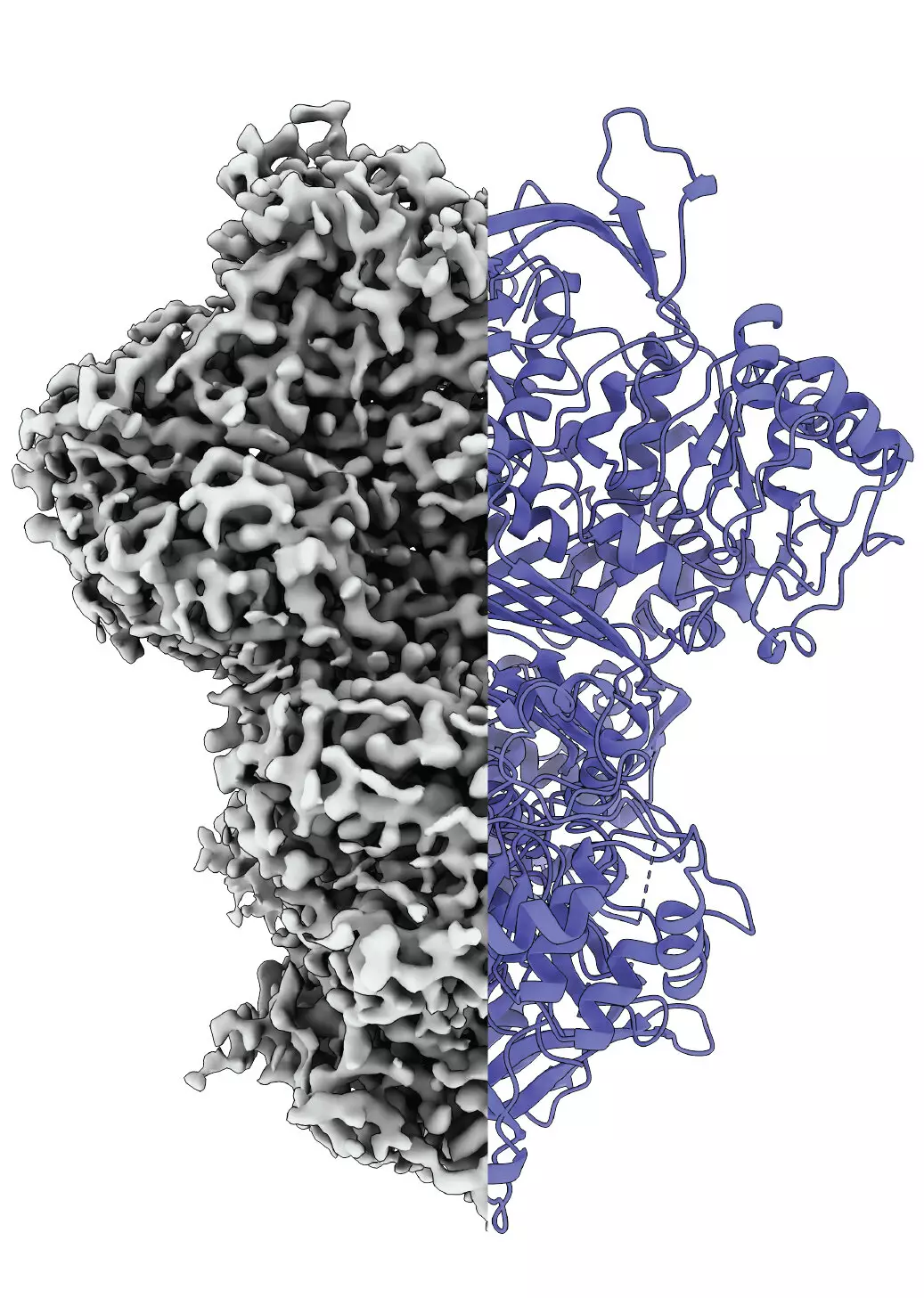
Proteins are molecular workhorses that govern numerous biological functions, ranging from metabolic regulation to cellular response mechanisms. One particularly important protein, myo-inositol-1-phosphate synthase (MIPS), plays a pivotal role in the biosynthesis of inositol, often affectionately referred to as vitamin B8. Although it is synthesized by the body and typically not classified as a vitamin, its significance in various metabolic pathways cannot be understated. Recent scientific endeavors at Martin Luther University Halle-Wittenberg (MLU) and the National Hellenic Research Center have unveiled fascinating insights into how MIPS changes its internal structure when activated. This transformation is crucial for a deeper understanding of protein functionality, particularly for those proteins that exhibit inherent structural disorder.
Integral to the field of biochemistry is the fundamental principle stating that the functionality of a protein is invariably linked to its structure. If even a minor distortion occurs at a crucial site within the protein, it may cease to perform its necessary biological functions. This relationship is significant in the context of human health, as dysfunctional proteins are often implicated in a range of diseases. However, the landscape of protein structure is not as straightforward as it seems. Many proteins, including MIPS, present with structural malleability and do not possess a static form. This disordered state makes them challenging subjects for analytical scrutiny, as their conformations may shift depending on the external environment and specific conditions.
Traditional approaches in protein research often isolate proteins from their biological context before analysis. While this method can yield informative data, it fails to account for the dynamic interactions and behaviors that occur in a natural environment. To address this challenge, Professor Panagiotis Kastritis and his team pioneered an innovative methodology that allows researchers to observe proteins in near-native conditions. Utilizing cryo-electron microscopy, they studied samples from the fungus Thermochaetoides thermophila, a model organism widely used in biochemical research. This advanced imaging technique unveiled the multifaceted nature of MIPS, revealing it exists in at least three distinct states: an unordered state, an ordered state, and an intermediate state.
Among the findings, the characterization of the third, intermediate state of MIPS has inspired curiosity. While the researchers have yet to elucidate its exact purpose, hypotheses abound. One possibility is that this transient state aids in water absorption, thereby facilitating subsequent biochemical reactions. Alternatively, it may serve a purpose that has yet to be explored fully. This vacancy in our understanding highlights the complexity of biochemical processes, emphasizing the need for further research to discern the behavioral patterns of proteins with dynamic structures.
The implications of Kastritis’s research extend beyond MIPS, as the study also examined over 340 related isomerases. Notably, the data indicated a similar structural behavior among these proteins, suggesting that the introductory findings concerning MIPS may apply to a wider class of proteins. Gaining insights into the functional mechanisms of these proteins could potentially pave the way for novel therapeutic interventions. Enriching our knowledge of metabolic pathways, coupled with insights into the proteins that facilitate these processes, could create opportunities for groundbreaking medical advancements.
The contributions of Kastritis and his team mark a significant advance in the understanding of protein dynamics, particularly for those classified as isomerases. As they continue to unravel the complexities associated with MIPS and its analogs, the pursuit of knowledge in protein functionality promises to yield rewarding developments in biochemistry and medicine. With greater understanding comes the potential for innovative treatments targeting protein-related ailments, reaffirming the impact of foundational research on future medical solutions. This ongoing exploration into the dynamic architecture of proteins represents not merely a scientific achievement but a critical avenue toward improving human health and addressing the challenges posed by various diseases.