Aluminum oxide, also referred to as alumina or corundum, has long been recognized as a crucial material in various industrial applications ranging from electronic components to catalysts. As a highly effective insulator, its properties make it a staple in many technological advancements. Yet, despite its importance, an intricate knowledge of its surface atomic structure has remained elusive, posing significant challenges in applications such as catalysis. Recent research conducted by a team from TU Wien and the University of Vienna has finally provided clarity to this complex puzzle. This study not only uncovers the details of aluminum oxide’s surface structure but also offers implications for broader material science.
Understanding the atomic arrangement of surfaces is critical for grasping how chemical reactions occur. Aluminum oxide plays a vital role in catalytic processes — substances that accelerate chemical reactions without being consumed by them. The working mechanism often hinges on how atoms are positioned at the surface level, where reactions actually take place. Despite the substantial insulating properties of alumina, hindrances in experimental approaches have kept researchers from definitively determining the surface structure for many years, which contributed to aluminum oxide being cataloged as one of the “three mysteries of surface science” in 1997.
Technological Endeavors Behind the Discovery
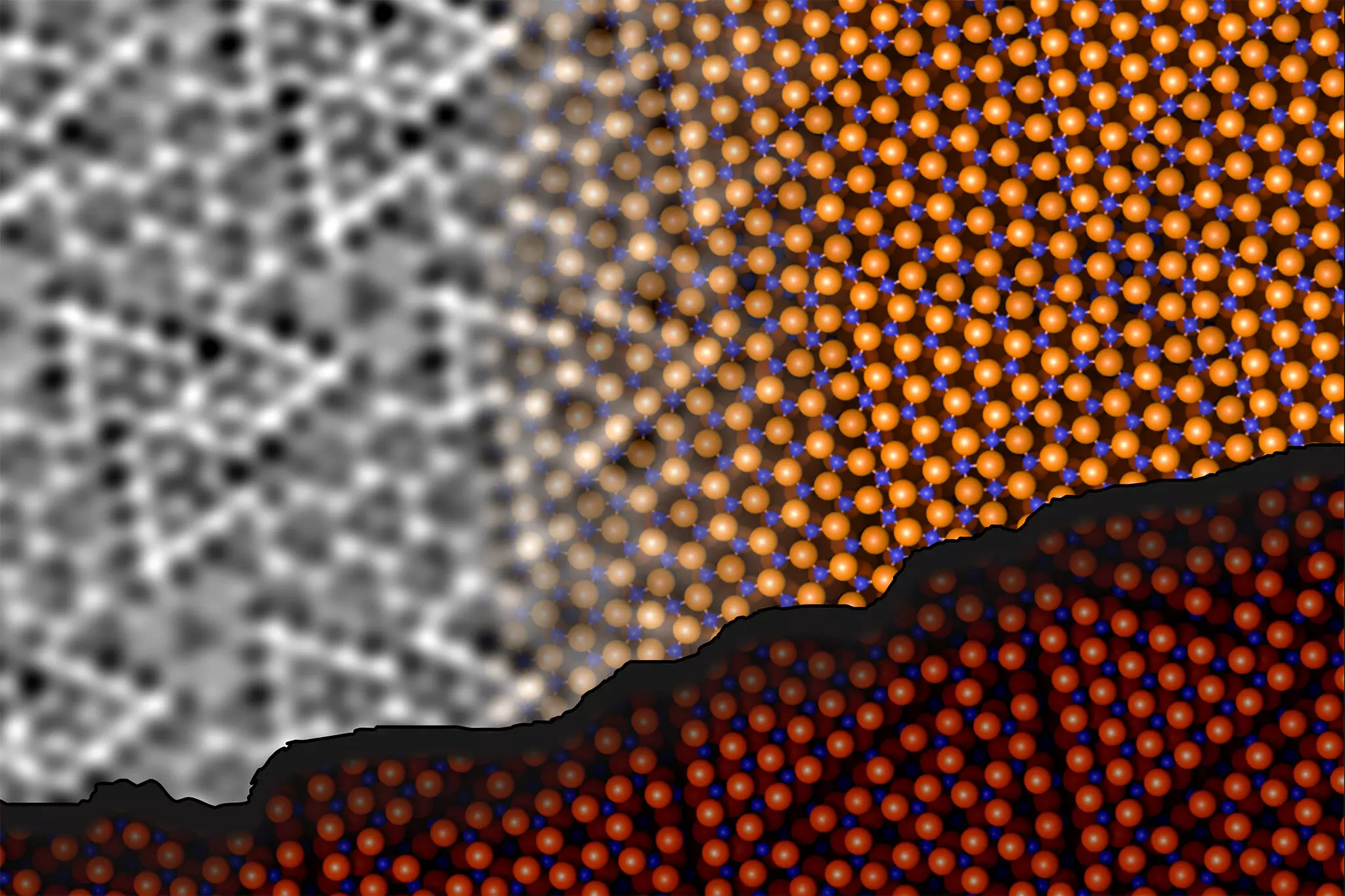
The innovative work carried out by Jan Balajka, Ulrike Diebold, and their team utilized noncontact atomic force microscopy (ncAFM) to unravel the complexities of aluminum oxide’s surface. This method works by scanning a finely tuned tip close to the surface without direct contact, allowing the generation of precise images. The evaluation of molecular interactions through slight changes in frequency of the tuning fork provided foundational data on the spatial arrangement of atoms on the surface.
Johanna Hütner, a key member of the research team, emphasized an ingenious technique involving the control of a single oxygen atom attached to the tip of the microscopy apparatus. This clever adaptation enabled researchers to identify not only where atoms were located but also their chemical identities — a pivotal advancement that allowed for the visualization of aluminum and oxygen atoms distinctly.
Rearrangement and Bond Formation: A New Understanding
Upon detailed examination, the research revealed a significant finding: the restructuring of the first two atomic layers at the surface facilitates aluminum atoms’ penetration into the alumina matrix. This helps them form bonds with deeper oxygen atoms, contradicting previous theories regarding atomic ratios. The remarkable stability achieved through this process suggests a dynamic adjustment of atomic arrangements that can profoundly affect material properties.
Prior beliefs posited that the aluminum to oxygen ratio was variable; however, the new findings indicate it remains constant even as the surface atomic structure changes. This insight opens new avenues for research into how surface interactions influence overall material behaviors.
A cornerstone of this study lies in the integration of machine learning techniques with traditional computational methods. The intricate task of creating a three-dimensional model of the aluminum oxide surface required reconciling the complex surface restructuring with the underlying crystal integrity. Andrea Conti, who spearheaded the computational modeling efforts, noted that the application of state-of-the-art algorithms enabled a significant examination of various atomic arrangements, facilitating the development of a stable model.
As this research illustrates, the collaboration of experimental findings and computational analysis can yield potent insights that challenge previously held beliefs. The meticulous work not only deciphers the structure of aluminum oxide but also establishes design principles that could extend to a wide array of materials.
Implications and Future Prospects
The ramifications of this breakthrough are immense for various fields such as catalysis, electronics, and materials science. By elucidating the structural details of aluminum oxide, researchers can enhance the efficiency of catalytic processes and improve material durability. The study serves as a springboard for future advancements, enabling more robust experimental designs and inspiring further investigations into the mysterious world of surface science.
The ongoing quest to unravel the mysteries of materials such as aluminum oxide underscores the importance of interdisciplinary research. The collective efforts of chemists, physicists, and computational scientists have confirmed that when we break down established boundaries, the scientific community can move toward significant breakthroughs that benefit multiple sectors.