As the world grapples with the consequences of climate change, the transition to cleaner energy sources becomes imperative. Hydrogen energy stands out due to its green, low-carbon attributes and high calorific value. Positioning itself as a solution to the global energy crisis, hydrogen can be produced through various methods, with electrochemical water splitting being one of the most promising techniques. However, the efficiency of this conversion process is hampered by the sluggish kinetics of the oxygen evolution reaction (OER), which takes place at the anode during electrolysis. To unlock the full potential of hydrogen energy, it is essential to enhance the efficiency of OER, making the development of advanced catalysts a priority in contemporary research.
At the heart of this energy transformation challenge lies the OER, a reaction that demands significant energy input due to its complex kinetics. One promising pathway to overcome the current inefficiencies is the use of catalysts—substances that accelerate reactions without being consumed. Among various catalyst designs, single-atom catalysts (SACs) have emerged as a revolutionary class, wherein isolated metal atoms distributed across a support material can dramatically enhance reaction rates. The performance of SACs is fundamentally influenced by the density of these single atoms on the catalyst’s surface. Increased atom density reduces the interatomic distance, fostering neighboring synergetic interactions that optimize the adsorption behavior of reaction intermediates.
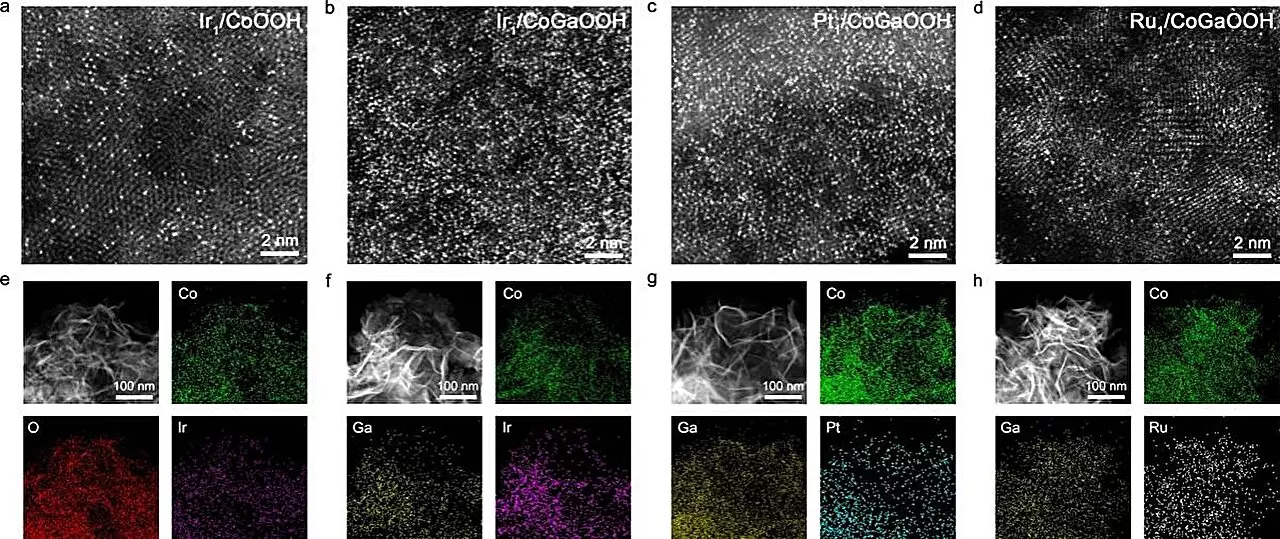
Recent advancements led by a research team under the guidance of Prof. Bao Jun at the University of Science and Technology of China (USTC) have illuminated new pathways to enhance OER efficiency through the use of cobalt-based, oxide-supported high-density Iridium (Ir) single-atom catalysts. Their innovative approach involved integrating Gallium (Ga) atoms into the cobalt oxide structure to manipulate the electronic characteristics of the anchoring sites for the single atoms. This strategic engineering resulted in a substantial enhancement of the bonding strength between oxygen-defect sites and the Ir single-atom precursors, thereby facilitating the construction of high-density SACs.
The findings of their research, published in the esteemed journal Angewandte Chemie, illustrate significant gains in catalytic performance. The high-density Ir single-atom catalyst, designated as Nei-Ir1/CoGaOOH, displayed an impressive overpotential of just 170 mV at a current density of 10 mA cm-2, coupled with outstanding stability under continuous operation for over 2000 hours. Furthermore, when subjected to harsher conditions, such as a current density of 1 A cm-2 in an alkaline medium, the catalyst maintained stability for over 50 hours, demonstrating remarkable durability and effectiveness.
A pivotal aspect of this research involves understanding the mechanisms that contribute to the catalytic success of high-density SACs. In situ Raman spectroscopy provided insights into the structural integrity of the catalysts during the OER process, confirming their stability throughout experiments. Contrary to traditional assumptions that attribute performance enhancements primarily to electronic structure optimizations, the study revealed that neighboring synergetic interactions among high-density single atoms play a more crucial role. By stabilizing particular reaction intermediates, such as *OOH, through additional hydrogen bonding interactions, the catalyst reduces energy barriers for the OER, significantly improving overall performance.
The implications of this research extend far beyond academic intrigue; they pave the way for future advancements in electrochemical catalysts. By elucidating the underlying mechanisms of high-density SACs, the USTC team has provided invaluable insights that could steer the development of more efficient catalysts not only for hydrogen production but across various electrochemical applications. The findings underscore the importance of strategic atom positioning and engineering in achieving high-performance catalysts, representing a notable step towards sustainable energy solutions.
As the world moves towards an era increasingly reliant on hydrogen as a clean source of energy, breakthroughs like those achieved by Prof. Bao Jun’s team are vital to realizing the efficiencies and economic viability required for widespread adoption of hydrogen technologies. The contribution of such innovative research holds the promise of overcoming the barriers that currently hinder OER efficiency, ultimately supporting a sustainable energy future.