In the pursuit of sustainable manufacturing, the development of efficient methods for transforming biomass into essential chemical precursors has gained immense significance. Researchers at Kyushu University have made an intriguing discovery that highlights the capabilities of a zeolite material, Na-ZSM-5, in this domain. Their findings, published in the *Chemical Engineering Journal*, suggest that using microwave heating could revolutionize the chemical conversion processes that underpin industries ranging from plastics to pharmaceuticals. This breakthrough presents a potent alternative to traditional methods, aiming to mitigate energy consumption and environmental impact in the chemical sector.
Historically, the chemical industry has relied heavily on high-energy processes, particularly the reforming of naphtha, to synthesize fundamental chemicals. While effective, this method is notorious for its substantial energy demands, primarily due to the exorbitant temperatures—typically between 500°C and 600°C—required to facilitate chemical reactions. Furthermore, this conventional approach yields significant carbon dioxide emissions, contributing to environmental degradation.
Alternative sources, such as cooking oil waste and microalgal oils, provide promising avenues for more environmentally-friendly chemical synthesis. These feedstocks can undergo catalytic cracking—a process that employs zeolites to convert simpler organic compounds into desired chemicals. However, the high thermal energy requirements of this method present additional hurdles, such as catalyst coking, which diminishes the catalysts’ lifespan and effectiveness.
The research team, led by Associate Professor Shuntaro Tsubaki, sought an innovative solution to these persistent challenges. Their approach involved utilizing microwave heating to power the zeolite catalysts. Unlike traditional heating methods, microwaves can penetrate directly into materials, allowing for precise and selective energy delivery. This capability results in reduced energy expenditure and improved reaction efficiency.
Tsubaki elaborates on the advantages: “Microwaves accelerate gas-solid catalysis by passing straight through the gas phase and heating the solid catalyst selectively.” This process generates localized hot spots within the catalyst beds, enhancing their overall performance and minimizing energy wastage.
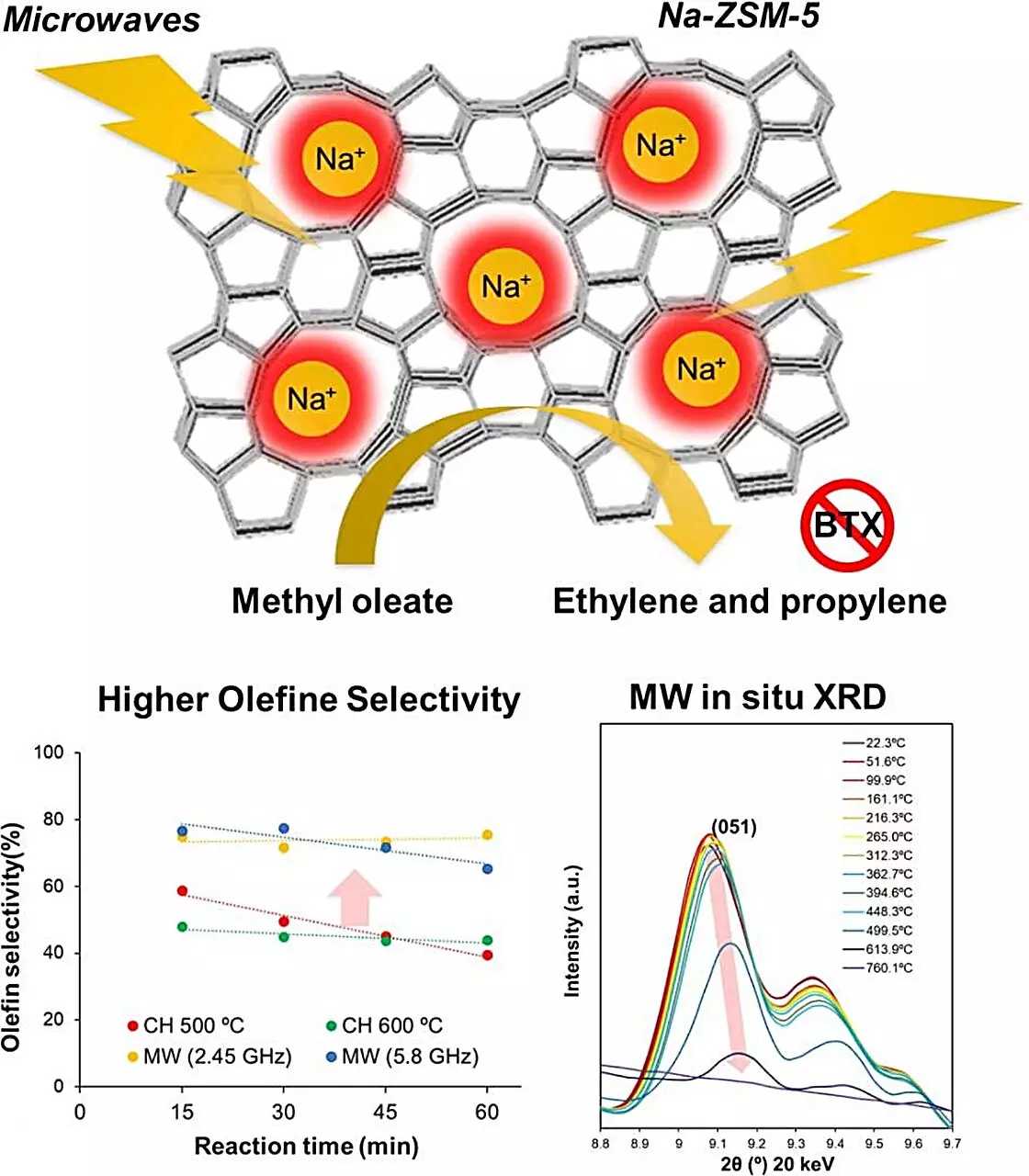
In their exploration, the team meticulously examined various zeolite catalysts, ultimately pinpointing Na-ZSM-5 as the most effective candidate for microwave heating applications. Through a combination of theoretical modeling and experimental validation, the researchers demonstrated that Na-ZSM-5 significantly outperformed its counterparts in converting methyl oleate into olefins—a crucial precursor for the synthesis of various compounds.
Astoundingly, under microwave heating, Na-ZSM-5 achieved an extraordinary conversion efficiency with a mere 1.3% carbon dioxide production and absent carbon monoxide. Furthermore, the olefin yield soared to four times that produced via conventional heating methods at the same temperature, primarily due to Na-ZSM-5’s remarkable selectivity towards olefins over other byproducts. Notably, the issue of coking was entirely eliminated, allowing for uninterrupted catalytic activity even as temperatures reached 600°C.
To understand the underlying phenomena driving these improved outcomes, the researchers conducted a thorough analysis of structural changes in the zeolite during microwave exposure. Fascinatingly, measurements revealed that localized temperatures within the crystal lattice exceeded 1000°C, while the bulk temperature remained stable at 500°C. This critical insight suggests that the extreme localized conditions facilitate the selective formation of olefins, thereby enhancing overall conversion efficiency and sustainability.
The implication of these findings extends beyond immediate applications; they herald a potential shift towards achieving sustainability goals within the chemical industry. Tsubaki notes the revolutionary potential of microwaves generated from renewable energy sources, reinforcing the alignment with global efforts to reduce the carbon footprint in chemical manufacturing processes.
As the team continues to refine their microwave-powered catalytic techniques, they aspire to boost both yield and energy efficiency while scaling their processes. The vision is clear: to usher in a new era characterized by sustainable approaches to chemical synthesis that align with the urgent need for environmental stewardship.
In sum, the research conducted by Kyushu University serves as a pivotal reminder of the transformative potential of embracing modern technology in traditional industries. Microwave-powered catalytic processes could very well reshape the landscape of chemical manufacturing, facilitating a greener and more efficient future for the industry. As scholars and practitioners work hand-in-hand to refine these methods, the hope is that more sustainable solutions can emerge to meet the diverse needs of today while safeguarding the planet for future generations.