Throughout history, the craft of blacksmithing has played an indispensable role in human technological advancement. From the Bronze and Iron Ages, blacksmiths discovered a pivotal phenomenon: the process of deforming metal through techniques like bending and hammering leads to an increase in strength. This integral method, known as work hardening or strain hardening, remains relevant today and is foundational in industries such as metallurgy and manufacturing. Contemporary applications of work hardening can be observed in the production of essential components, ranging from automotive frames to electrical wiring. Despite its widespread usage, the intricate details of how this process operates at the atomic level have eluded scientists—until now.
Breakthrough Research at Harvard
A pioneering team from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS) has accomplished what many considered unfeasible: observing the complex mechanisms of work hardening in real time. This groundbreaking research, carried out at the Harvard Materials Research Science and Engineering Center (MRSEC), enables scientists to peer into the fundamental interactions that enhance material strength. Published in the prestigious journal Nature, the findings offer a comprehensive understanding of material behavior, paving the way for improved design and manufacturing processes.
Frans Spaepen, who serves as the John C. and Helen F. Franklin Professor of Applied Physics at SEAS, emphasizes the significance of this study. Noting that existing computer simulations of work hardening could greatly benefit from more precise knowledge about the underlying mechanisms, Spaepen’s research offers a vital real-time perspective that enhances our theoretical models.
Challenges in Observing Atomic Structures
Historically, observing the work hardening phenomenon in metals was problematic due to the limitations of electron microscopy. Scientists had the ability to assess the atomic structure of materials but were restricted to comparing conditions before and after deformation—not during the actual process. Previous research had already established that defects known as dislocations within the atomic structure create a network that leads to work hardening. However, the full spectrum of interactions among these defects remained a mystery.
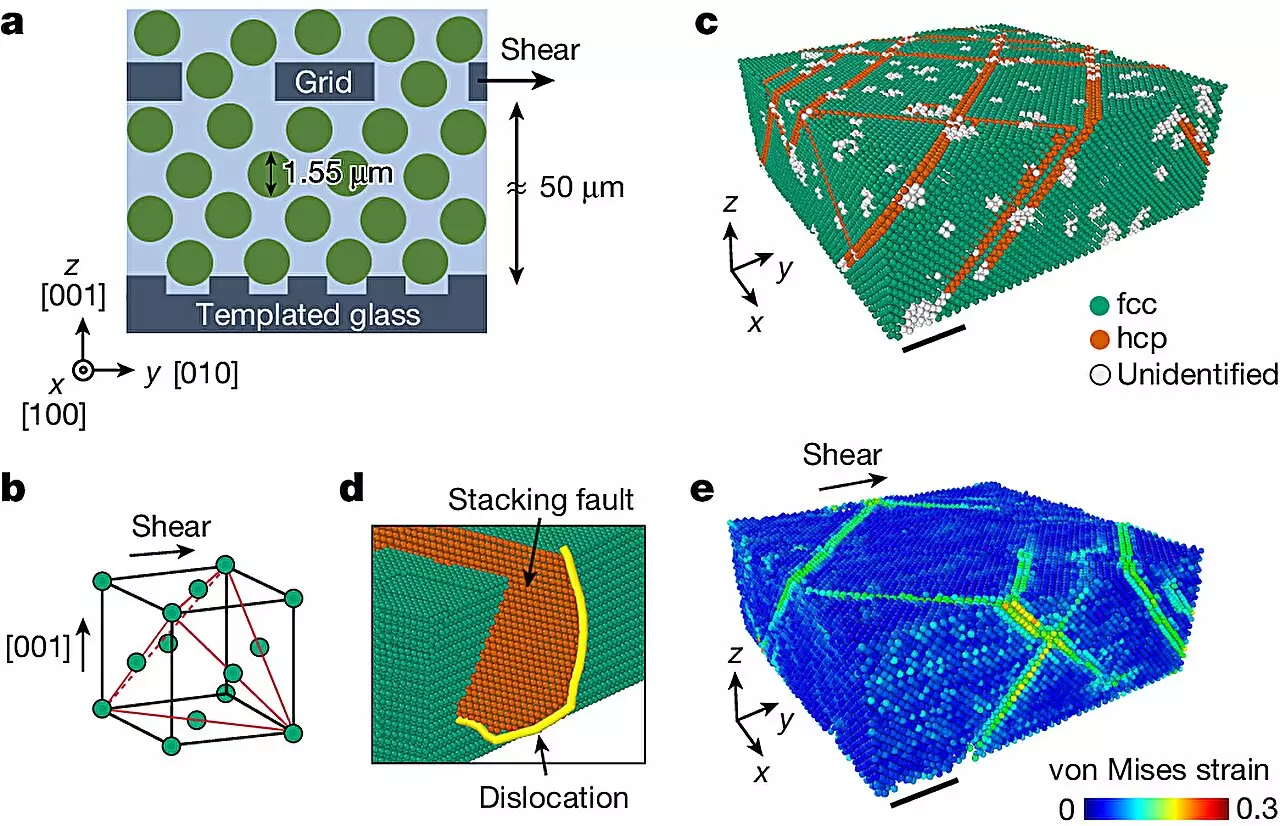
Addressing this gap, Ilya Svetlizky, a former postdoctoral fellow at SEAS, highlighted the importance of unraveling the interactions between defects that contribute to enhanced material strength. To explore this critical aspect, the research team shifted their focus from traditional metals to colloidal crystals—larger particles that can mimic atomic behavior without the same observational constraints.
Colloidal crystals, composed of particles approximately 10,000 times larger than atoms, possess the ability to spontaneously form a structured arrangement at high concentrations. Although these crystals are much softer than metals—often referred to as being 100,000 times softer than Jell-O—they provide a unique opportunity for insights into atomic systems. Researchers were able to cultivate colloidal crystals and utilize advanced confocal optical microscopy to track the movement of individual particles during deformation.
In a surprising revelation, the researchers discovered that these otherwise soft materials exhibited strong work hardening characteristics that surpassed even many traditional metals. By accounting for the size variation among particles, the colloidal crystals demonstrated an increased strength that outperformed typical metals like aluminum and copper. “We did not expect that hard-sphere colloidal crystals could be work hardened,” stated Seongsoo Kim, a graduate student supported by the MRSEC. This observation challenges preconceived notions about the limitations imposed by particle interactions in softer materials.
The most significant finding from this research was the realization that work hardening in colloidal crystals is primarily influenced by the geometry of the particles and the interactions among defects. As these crystals underwent deformation, the interactions, entanglements, and configurations of dislocation defects led to profound increases in strength—revealing a universal principle applicable to various materials.
This discovery signals a new direction in material science. David A. Weitz, Mallinckrodt Professor of Physics and Applied Physics and a co-author of the study, remarked on the broader implications of this work: “This research elucidates fundamental and universal insights into how materials can achieve greater strength. Despite their apparent softness, these materials undergo significant work hardening, making them some of the strongest substrates known.”
The research conducted by the Harvard team represents a turning point in our understanding of material behavior. By revealing the critical dynamics of work hardening in colloidal crystals, this study opens up new avenues for material applications and innovations. The implications extend far beyond academic curiosity; the insights gained from this line of inquiry have the potential to transform the manufacturing landscape and inspire the development of stronger, more resilient materials across various industries. As the world continues to face challenges requiring advanced materials, the findings from this study may provide the foundational knowledge needed for future breakthroughs in strength and functionality.