In a groundbreaking study, an international collaboration of chemists from the University of Hong Kong, Northwestern University, and Duke University has unveiled a novel supramolecular material aimed at enhancing hydrogen storage capacity. Published in the esteemed journal Nature Chemistry, this research responds to the longstanding challenge of effectively storing hydrogen—a potential clean energy source that has faced barriers in practical applications due to its storage inefficiencies. This article delves into the intricacies of the newly developed material and the implications of this advancement for the energy sector.
Hydrogen, while heralded for its eco-friendliness, poses significant challenges in terms of storage. Unlike conventional fuels such as gasoline, hydrogen requires substantially more space, complicating its adoption as a mainstream energy source. As global energy demands rise, the need for efficient hydrogen storage solutions has intensified. The research team’s approach centers around the use of porous organic crystals, which promise to not only compress hydrogen more effectively but also fulfill performance benchmarks set by the U.S. Department of Energy.
The benchmarks are particularly ambitious: achieving a storage capacity of at least 50 grams of hydrogen per liter of material while ensuring that the stored hydrogen accounts for a minimum of 6.5% of the total weight of the storage solution. Previous attempts to meet these objectives have often resulted in limited success; however, the newly developed supramolecular material surpasses these criteria by providing impressive storage metrics.
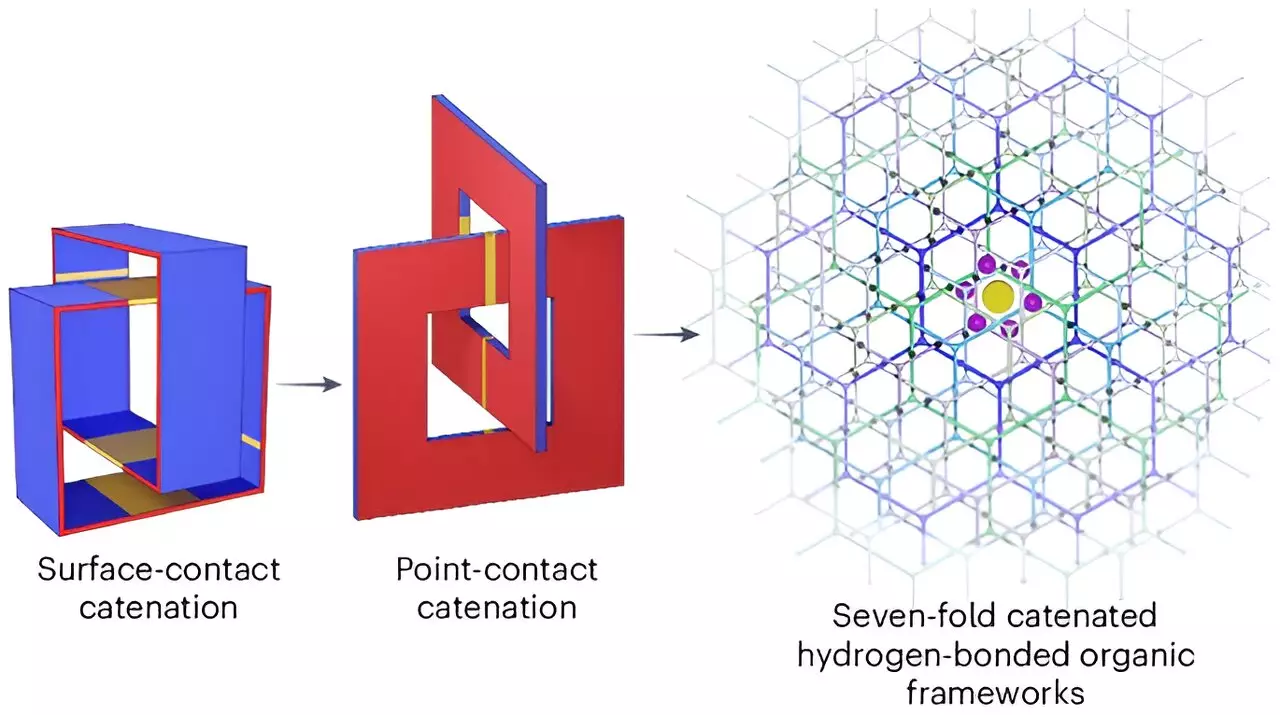
The researchers crafted the new material through the creation of organic molecules, interconnected in a honeycomb lattice structure. This unique configuration features precisely sized pores that are ideal for accommodating hydrogen molecules. The scientific principle behind the material’s efficacy lies in its ability to bond hydrogen atoms with the organic crystals, thereby maintaining structural integrity while facilitating efficient storage.
Testing revealed that this innovative storage material could encapsulate an impressive 53.7 grams of hydrogen per liter, with hydrogen contributing approximately 9.3% to the total weight. These results not only place the new material well within established storage targets but also signify a significant leap toward practical applications in hydrogen fuel technologies.
Despite these commendable advancements, there are notable hurdles that remain. Perhaps the most significant drawback of the newly developed hydrogen storage system is its requirement for cryogenic cooling. While this feature enhances the material’s storage capacity, implementing such cooling mechanisms can introduce additional complexities and costs, particularly in commercial settings. The potential for bulky refrigeration equipment could hinder widespread adoption, underscoring the need for further innovations that could streamline this process.
The promising results of this research mark a crucial step in the journey towards more viable hydrogen storage solutions. As the world seeks to transition to cleaner energy alternatives, continued exploration and refinement in materials such as this supramolecular substance will be essential. The collaboration between leading universities signifies a collective effort to tackle not only the technological challenges but also to pave the way for a more sustainable energy future. As these technologies evolve, the dream of utilizing hydrogen as a prevalent energy source may edge closer to reality.