The discovery and implementation of click chemistry has significantly transformed the landscape of chemical synthesis by promoting high selectivity, yield, and efficiency in various applications. It has established a reputation for facilitating the rapid connection of diverse molecules, making it a favored method across sectors like pharmaceuticals, materials science, and chemical biology. Despite its successes, the promise of click reactions has often been shadowed by the need for toxic reagents, particularly in the synthesis of key compounds such as sulfonyl fluorides. A recent breakthrough in this area highlights a novel method that sidesteps these hazardous materials, leading to safer and more sustainable practices in chemical synthesis.
A Greener Pathway to Sulfonyl Fluorides
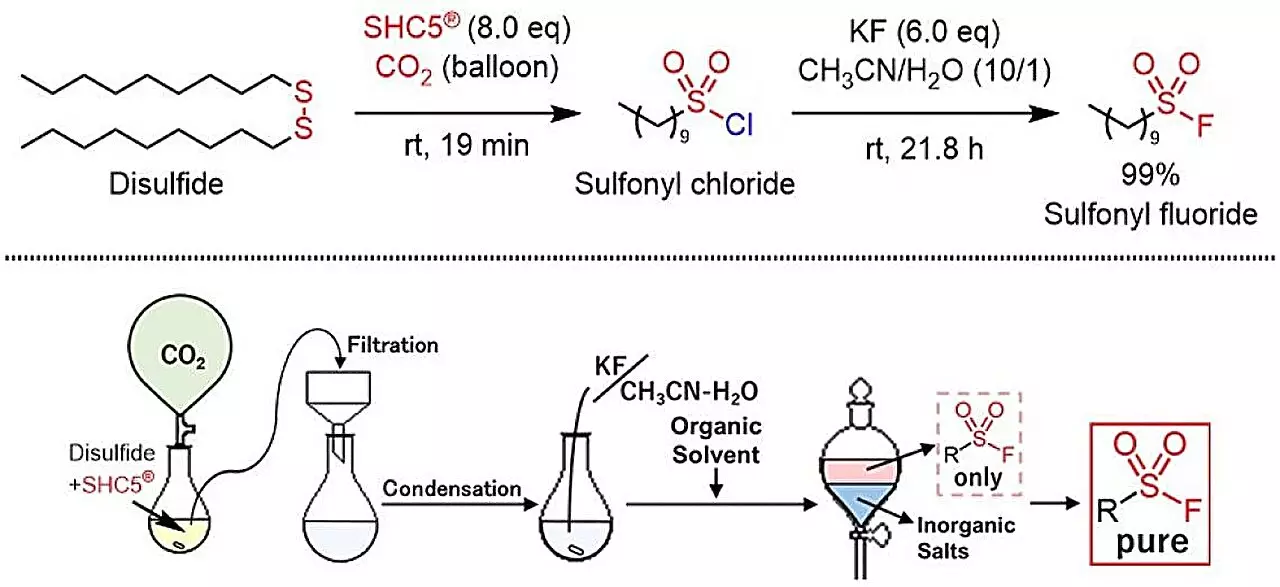
The study published in ACS Sustainable Chemistry & Engineering showcases a pioneering approach to producing sulfonyl fluorides by utilizing SHC5 (a reactive thiol) and potassium fluoride (KF). This innovative reaction converts thiols and disulfides into sulfonyl fluorides while only producing benign byproducts—sodium chloride and potassium chloride. This not only represents an advancement in synthetic methodology but also aligns with the growing emphasis on green chemistry principles, which advocate for processes that minimize waste and toxicity.
Previously, methods for synthesizing sulfonyl fluorides relied heavily on the use of highly toxic substances, such as sulfur difluoride (SO2F2) or potassium hydrogen fluoride (KHF2). These hazardous conditions compromised the safety and environmental stewardship essential in modern chemistry. The new synthesis method introduced in this research significantly mitigates these risks, ensuring that the resultant sulfonyl fluorides can be produced in a manner that is both effective and harmless to the environment.
Broad Scope and Simplification of Synthesis
One of the major advantages of this new method is its broad applicability. The ability to create various sulfonyl fluorides comprising aromatic, aliphatic, and heterocyclic groups expands its usefulness in many chemical sectors. This versatility is a crucial element for researchers and industries seeking to develop novel compounds for applications ranging from drug discovery to novel materials. Furthermore, the straightforward nature of this synthetic protocol positions it well for low-cost and scalable production, making it an attractive option for commercial use.
Chemists Masayuki Kirihara, Shinobu Takizawa, and Mohamed S. H. Salem underscore the importance of developing new synthetic strategies that not only yield effective chemicals but also do so responsibly, in light of increasing global sustainability goals. Their research signifies a step forward in meeting the Sustainable Development Goals (SDGs) that advocate for eco-friendly practices in industrial chemistry.
As industries and researchers continue to prioritize sustainability, methods like this innovative green synthesis of sulfonyl fluorides could become mainstream in chemical manufacturing. The implications of this research extend beyond mere chemical production; they underscore a broader commitment to environmental stewardship and safe practices in science. With growing pressure to pivot towards sustainable methods, it is essential that the scientific community embraces methodologies that not only fulfill the needs of today but also safeguard the planet for future generations. This recent advancement in click chemistry stands as a testament to how innovation can drive both efficiency and responsibility in synthetic processes, shaping the future of the chemical industry.