The atmosphere is a complex system that continually surprises researchers with its intricate chemical processes. A significant breakthrough has emerged from the Leibniz Institute for Tropospheric Research (TROPOS) located in Leipzig. For the first time, scientists have demonstrated the existence of sulfurous acid (H₂SO₃) in the gas phase under atmospheric conditions. This finding, published in the journal Angewandte Chemie, challenges longstanding assumptions about sulfurous acid’s formation and stability in the atmosphere, particularly when compared to its more recognizable counterpart, sulfuric acid (H₂SO₄).
The Historical Context of Sulfurous Acid
Historically, sulfurous acid has been seen as a challenging compound to isolate and study. Textbooks have stipulated that H₂SO₃ could only form in an aqueous solution of sulfur dioxide (SO₂), asserting that its independent existence was nearly impossible. Efforts to detect H₂SO₃ experimentally, particularly in aqueous SO₂, have yielded limited success. Prior to this research, detection was only achieved through specific conditions in a mass spectrometer by Helmut Schwarz’s team in 1988, indicating an incredibly brief existence under vacuum—a mere 10 microseconds. Such a short lifespan suggested that H₂SO₃ might not play a substantial role in the atmospheric chemistry.
The Experimental Approach and Results at TROPOS
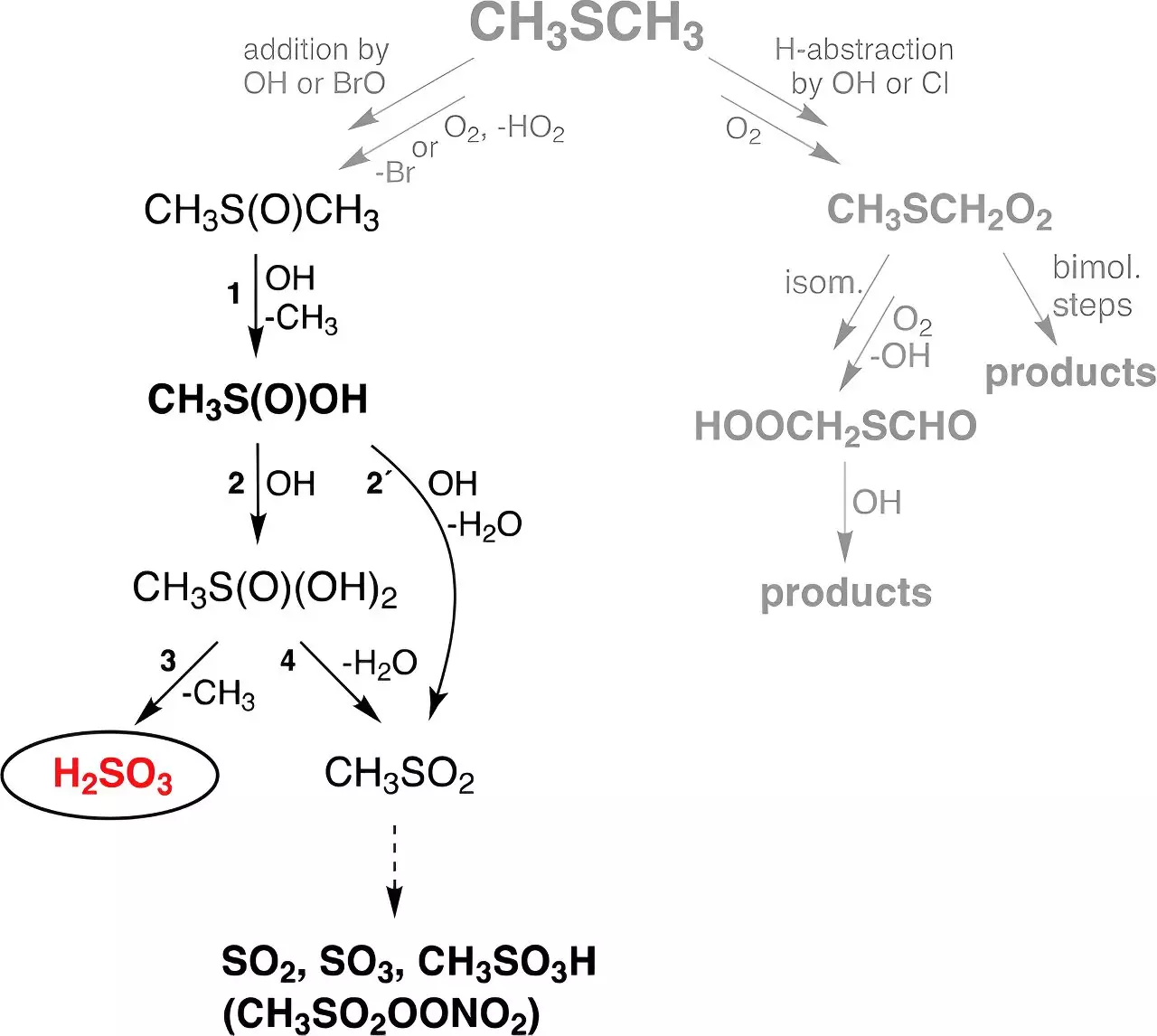
Despite these challenges, researchers at TROPOS embarked on an innovative experimental journey. They aimed to explore the reactions involving dimethyl sulfide (DMS)—a biogenic sulfur source predominantly produced by marine organisms. The study posited that H₂SO₃ could form from the reaction of DMS with hydroxyl (OH) radicals generated from ozone and water vapor under ultraviolet (UV) radiation. The experiments were conducted in controlled flow reactors mimicking atmospheric conditions, leading to a noteworthy finding: sulfurous acid maintained a stable presence for up to thirty seconds, regardless of the humidity levels.
Dr. Torsten Berndt, one of the leading researchers, expressed his astonishment at this revelation, stating that observing distinct H₂SO₃ signals in the spectrometer was remarkable for a compound once deemed nearly non-existent. This landmark discovery not only solidified the existence of H₂SO₃ in the atmosphere but also illuminated new avenues for understanding the sulfur cycle within atmospheric chemistry.
The implications of these findings extend far beyond a simple laboratory exercise. Utilizing a global chemistry-climate model, the researchers estimated that approximately 8 million tons of H₂SO₃ are generated annually on a global scale. This is dwarfed by the 200-fold increase in mass compared to the direct production of sulfuric acid from DMS. Such a volume of sulfurous acid formation in the atmosphere could significantly alter our understanding of sulfur dynamics and how these compounds interact with other atmospheric constituents.
Dr. Andreas Tilgner and Dr. Erik Hoffmann, who contributed to the modeling efforts, emphasized that the newly identified formation pathway enriches our understanding of the atmospheric sulfur cycle. This is critical as it may influence not only the chemical reactions occurring in the atmosphere but also the broader implications on climate models and environmental policies.
The discovery inevitably raises numerous questions that beckon further investigation. Notably, while H₂SO₃ appears to be stable enough to be detected in the gas phase, its lifetime and reactivity concerning trace atmospheric gases remain enigmatic. Researchers are particularly interested in how H₂SO₃ interacts with water vapor, and what implications those reactions could have on overall atmospheric chemistry.
Dr. Berndt calls for more optimized experimental designs to clarify the role and significance of sulfurous acid further. As detection methods evolve, the potential to uncover more about such transient species may yield unprecedented insights into atmospheric processes and even broader chemical contexts.
The detection of sulfurous acid in the atmosphere marks a pivotal moment in atmospheric chemistry, showcasing the importance of using advanced detection techniques and innovative experimental setups. As scientists continue to push the boundaries of what is known, this discovery encourages a reevaluation of theoretical chemical pathways that were once thought inaccessible. The evolving understanding of H₂SO₃ serves as a reminder that the more we learn, the more questions arise, opening new avenues for exploration in the atmospheric sciences.