The efficiency of batteries is critically dependent on the interaction that occurs at the interface between electrodes and electrolytes. In recent years, the scientific community has focused considerable attention on improving this interface, especially with regards to lithium-metal batteries (LMBs). These batteries are seen as a next-generation solution that could resolve many of the limitations faced by traditional lithium-ion batteries (LiBs). They pivot away from the commonly used graphite-based anodes and leverage lithium metal anodes instead. This transition opens avenues for potential increases in energy density and faster recharge capabilities. However, the quest for LMBs to enhance battery performance faces significant challenges.
One of the foremost obstacles in the development of LMBs is the formation of lithium dendrites during the charging process. Dendrites are essentially tree-like protrusions made of lithium metal that develop on the anode surface, which not only compromises the efficiency of the battery but also presents serious safety risks, including overheating and potential fire hazards. Moreover, issues related to manufacturing costs and low Coulombic efficiency further hinder the commercialization of LMBs. These challenges necessitate innovative research efforts that can address the underlying causes of these limitations and facilitate the advancement of reliable and high-performance battery systems.
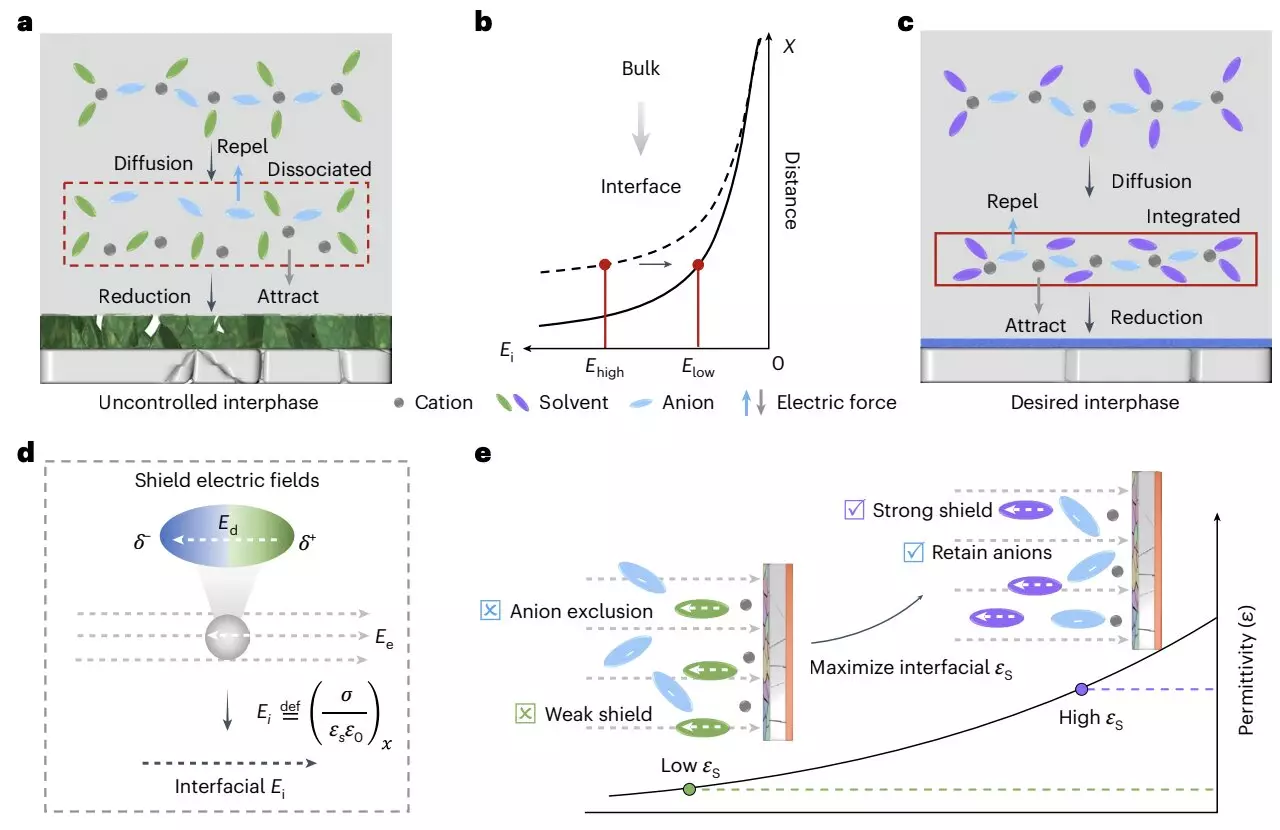
Another contributing factor to the inefficiency of LMBs is the electrode/electrolyte interface, which is influenced by the surrounding dielectric environment. Despite extensive research directed at improving the chemistry and architecture of these batteries, many studies overlook the complex role that dielectric materials play in stabilizing or destabilizing the battery interface.
Researchers at Zhejiang University, alongside collaborators from other Chinese institutes, recently conducted an important study aimed at addressing these critical issues. Their findings, published in *Nature Energy*, propose a novel dielectric protocol designed to enhance the stability and performance of LMBs. As co-author Xiulin Fan highlighted, the push for better batteries is not merely about improving efficiency; it also plays a vital role in advancing toward a low-carbon economy.
The researchers laid out a framework that considers the influence of the interfacial electric field—dynamically modulated through the dielectric materials used in the battery. By promoting a favorable dielectric environment, they were able to enhance cation-anion coordination, thus facilitating the development of the solid-electrolyte interphase (SEI) essential for battery performance. The SEI represents a protective layer that forms on the electrode during battery operation, influencing battery life and function.
Mechanism Behind the Dielectric Protocol
The novel approach involves the utilization of high-dielectric-constant solvents intended to shield cation-anion pairs from dissociation caused by the interfacial electric field. In this ideal setup, an anion-rich region emerges near the electrode-electrolyte interface, which allows for effective anion decomposition and, ultimately, enhanced interfacial chemistry for lithium deposits. The research team posited that maintaining the oscillation amplitude of cation-anion pairs can mitigate the chances of electrolyte decomposition and lower the surface impedance.
Through meticulous experimentation, the researchers demonstrated that using an ultra-lean electrolyte in lithium-metal pouch cells yielded an impressive energy density of 500 Wh/kg. This revelation holds significance not only for the feasibility of LMBs but also for the future of battery technologies that aim for longer-lasting, high-density solutions suitable for various applications.
Implications and Future Directions
As the demand for advanced battery technology continues to surge—especially in the electric vehicle and energy storage sectors—the insights garnered from this research could prompt further innovations in electrolyte design. The findings underscore the versatility and adaptability required in creating more efficient battery architectures. Other research teams might adopt this dielectric-mediated methodology to explore even more promising electrolytes for LMB applications.
Nevertheless, the path forward must be approached with caution. The explosive potential associated with high-energy-density lithium-metal batteries poses serious safety concerns that must not be overlooked. As the technology matures, a comprehensive strategy for managing risks while enhancing performance must be developed, ensuring that LMBs become a viable option for the clean energy revolution.
The intersection of dielectric studies with lithium-metal battery technology opens new avenues for creating safer, higher density batteries that could propel the next generation of electric power storage solutions. With continued research and innovation, LMBs may well fulfill their promise of revolutionizing energy storage and consumption.