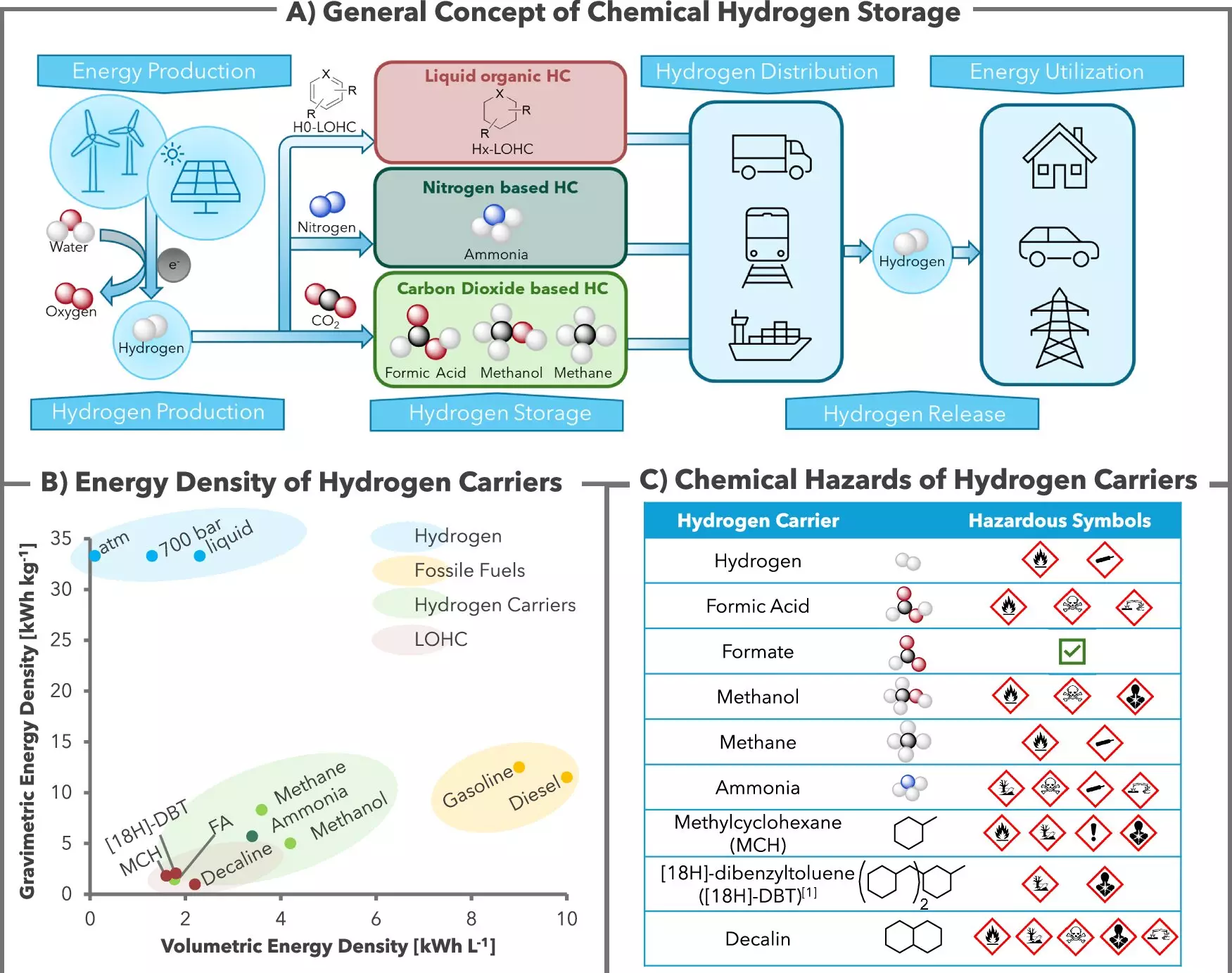
The quest for efficient and secure hydrogen storage is gaining momentum as the world pivots towards sustainable energy solutions. Researchers from the Leibniz Institute for Catalysis (LIKAT) in Rostock, Germany, in collaboration with H2APEX, have developed a revolutionary system that chemically binds hydrogen gas to potassium bicarbonate—a simple and easily attainable compound. Their findings, published in *Nature Communications*, indicate a promising step forward in harnessing hydrogen as a vital component of the energy transition.
Hydrogen is increasingly celebrated as a cornerstone of the future energy landscape. However, its volatile nature and tendency to ignite pose significant challenges for safe storage. The breakthrough achieved by the research team lies in their development of a homogeneous catalyst system that allows hydrogen to safely connect with potassium bicarbonate. This chemical reaction creates a stable compound—formate, a non-toxic salt derived from formic acid—ensuring that hydrogen can be stored securely and released whenever needed.
The pivotal role of the ruthenium catalyst in this process is crucial yet unobtrusive; it facilitates the reaction but does not undergo any degradation. This characteristic allows for the formation of a reversible reaction, which means that hydrogen can be extracted from formate effortlessly, reinstating the compound’s utility.
The described system operates efficiently at moderately elevated temperatures (approximately 60°C) within a solution that integrates all required components—hydrogen, potassium bicarbonate, and the catalyst. By adjusting the pressure during hydrogen introduction, the system allows for either the binding of hydrogen to bicarbonate or the reverse process, thereby releasing hydrogen. The ability to manipulate this reversible reaction emphasizes its appeal as an energy solution akin to battery technology, where energy can be stored and released on demand.
Dr. Henrique Junge, leading the research group, stresses the importance of this system as a game-changing technology for energy storage. The reactivity and stability of hydrogen storage in the form of formate present a less hazardous alternative to existing methods that typically employ methanol, ammonia, or methane for energy management.
The advantages of using formate over traditional hydrogen storage methods extend beyond mere toxicity. Formate can be securely contained in common plastic containers and transported much like liquid fuels such as milk or diesel. This characteristic positions it as a particularly suitable option for rural energy systems where localized renewable energy sources—like solar or wind—can generate excess hydrogen through electrolysis. The hydrogen can then be stored as formate during surplus production and utilized when demand necessitates its release.
The synergy between potassium bicarbonate and formate creates an energy storage medium that is both simple and efficient. The ongoing collaboration between LIKAT and H2APEX aims to maximize hydrogen storage density and solubility, factors determined by the properties of the salts involved. After considering multiple candidates, potassium bicarbonate emerged as the optimal choice for creating a hydrogen-storing composite.
This innovative method of hydrogen storage is also noteworthy for its environmental implications. According to the researchers, the entire process is CO2-neutral, which contrasts sharply with conventional methods that typically result in CO2 emissions during hydrogen recovery. This new system actually sequesters CO2, maintaining it within the compound and thus offering a cleaner alternative that produces high-purity hydrogen ideal for use in fuel cells.
In practical demonstrations, the researchers successfully executed 40 cycles of hydrogen storage and release over a span of six months. The outcomes revealed that minimal amounts of ruthenium as a catalyst could generate an impressive total of 50 liters of hydrogen at an exceptional average purity level of 99.5%. These findings are encouraging and point to the feasibility of scaling up this technology for real-world applications.
With ambitions to transition from laboratory successes to commercial applications, H2APEX plans to build a demonstration unit based on these findings by the end of 2025. Leveraging the remarkable results of this research, the potential for this hydrogen storage technology to transform the energy sector looms large. Henceforth, the chemical symbol “H” may very well symbolize not just hydrogen, but hope—the promise of a greener, sustainable energy future. This pioneering work reflects a significant stride toward achieving essential advancements in renewable energy utilization, underscoring the critical role of collaboration in scientific innovation.