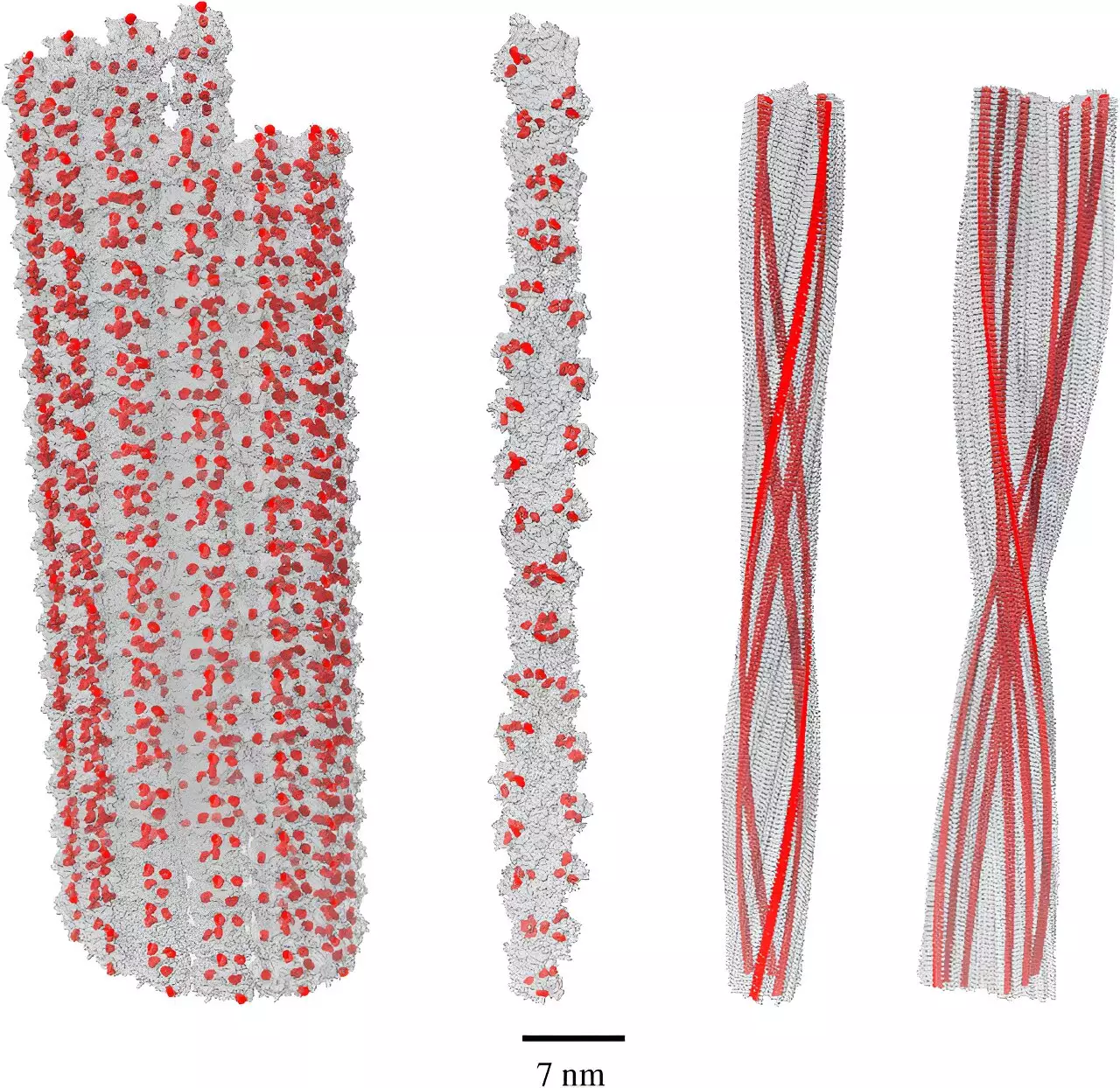
Alzheimer’s disease, one of the most enigmatic and devastating neurodegenerative disorders, has long puzzled scientists and healthcare providers alike. Central to this puzzle are amyloid fibrils—fibrous protein structures that have become a conventional target in Alzheimer’s research and treatment. Recent discoveries in the field of quantum biology, particularly concerning the unique properties of these amyloid fibrils, challenge long-held beliefs about their role in the onset and progression of the disease.
Traditionally, amyloid fibrils have been viewed as culprits in the development of Alzheimer’s, with numerous experimental therapies designed to reduce their presence or formation in the brain. Interestingly, many individuals with significant amyloid accumulation do not go on to develop dementia. This disparity raises critical questions about the biological functions of amyloid fibrils and their relationship with Alzheimer’s pathology. While the dominant narrative paints amyloid as a harmful factor, recent insights suggest that these structures might actually serve a protective role, complicating the simplistic view that has prevailed for decades.
Another significant factor in Alzheimer’s research is the concept of allostatic load, which refers to the cumulative wear and tear on the body due to chronic stress and oxidative reactions. Oxidative stress occurs when there is an imbalance between free radicals—high-energy particles that can potentially harm cells—and the body’s ability to neutralize them. Given this backdrop, understanding the interplay between amyloid fibrils and oxidative stress is vital. Recent studies have illuminated a novel mechanism through which amyloid fibrils can mitigate the effects of these damaging free radicals, primarily due to their high density of the amino acid tryptophan.
Researchers, led by Dr. Philip Kurian of Howard University’s Quantum Biology Laboratory, have investigated the phenomenon of single-photon superradiance in relation to tryptophan networks. This quantum effect allows molecules to absorb high-energy light particles and re-emit them at a lower, less harmful energy state. Kurian’s recent findings imply that amyloid fibrils have an astonishing capacity for this superradiant effect—up to five times greater than that of individual tryptophan molecules. This suggests a radical shift in our understanding of amyloid fibrils: rather than merely accumulating to cause harm, they might indeed act as a buffering system against cellular damage.
These findings compel us to rethink the traditional narrative surrounding Alzheimer’s disease. If amyloid fibrils can protect against oxidative stress rather than exacerbate it, this opens up new avenues for treatment and prevention. Instead of developing therapies solely aimed at reducing amyloid levels, future research may explore how to enhance these protective capacities or harness the underlying mechanisms for therapeutic benefit.
As Professor Lon Schneider from the USC California Alzheimer’s Disease Center articulates, the implications of Kurian’s work are profound. By positioning amyloid aggregation as a potential protective response, we are led to reconsider treatment approaches that have thus far proven unsuccessful. This emergent understanding encourages a significant paradigm shift, prompting researchers to explore the role of quantum dynamics within the complex web of biological systems.
Dr. Kurian emphasizes the importance of interdisciplinary approaches in unraveling the complexities inherent in biological systems. As he notes, the convergence of quantum physics, computational biology, and photophysics can yield insights that transcend traditional scientific boundaries. This perspective promotes a holistic understanding of life, challenging the compartmentalization that has plagued scientific endeavors.
The implications of this approach, as echoed by Mr. Hamza Patwa, a senior intern in Kurian’s lab, highlight the essence of modern scientific inquiry: integrating diverse fields to foster innovation and broaden our comprehension of complex phenomena.
The evolving narrative surrounding amyloid fibrils and their complex relationship with Alzheimer’s disease marks an essential turning point in research. As we delve deeper into the quantum mechanics that underpin biological responses, we must be willing to discard outdated assumptions that have dictated therapeutic strategies. Embracing these new paradigms can lead to more effective treatment avenues and, ultimately, a deeper understanding of the intricate workings of the human brain. The ongoing research into quantum effects in neurobiology not only has the potential to illuminate the mysteries of Alzheimer’s but may also reshape our grasp of consciousness itself, ushering in a new era of scientific discovery.