The urgent need for sustainable production methods in agriculture and energy has turned the spotlight on ammonia, a compound integral to global food security and an emerging contender as a zero-carbon fuel. Traditionally, ammonia is synthesized through the Haber-Bosch process, a method notorious for its high energy demands and significant carbon footprint, contributing approximately 1.8% to global CO2 emissions. Such inefficiencies call for innovative approaches that not only decrease environmental impact but also enhance the viability of ammonia as an alternative energy source. Recent advancements in electrochemical nitrate reduction reactions (eNO3RR) have shown promise in addressing these challenges, particularly through research centered around spinel cobalt oxides (Co3O4).
The eNO3RR process offers a dual benefit: it transforms nitrate waste—an environmental pollutant—into ammonia and simultaneously proposes a greener route for ammonia production. Researchers have directed their efforts toward discovering catalysts that can facilitate this reaction effectively. A notable study published in the prestigious journal ACS Nano has taken significant steps in this direction, showcasing the potential of Co3O4 catalysts. These compounds are not only cost-effective but also exhibit remarkable activity and selectivity for this transformative reaction.
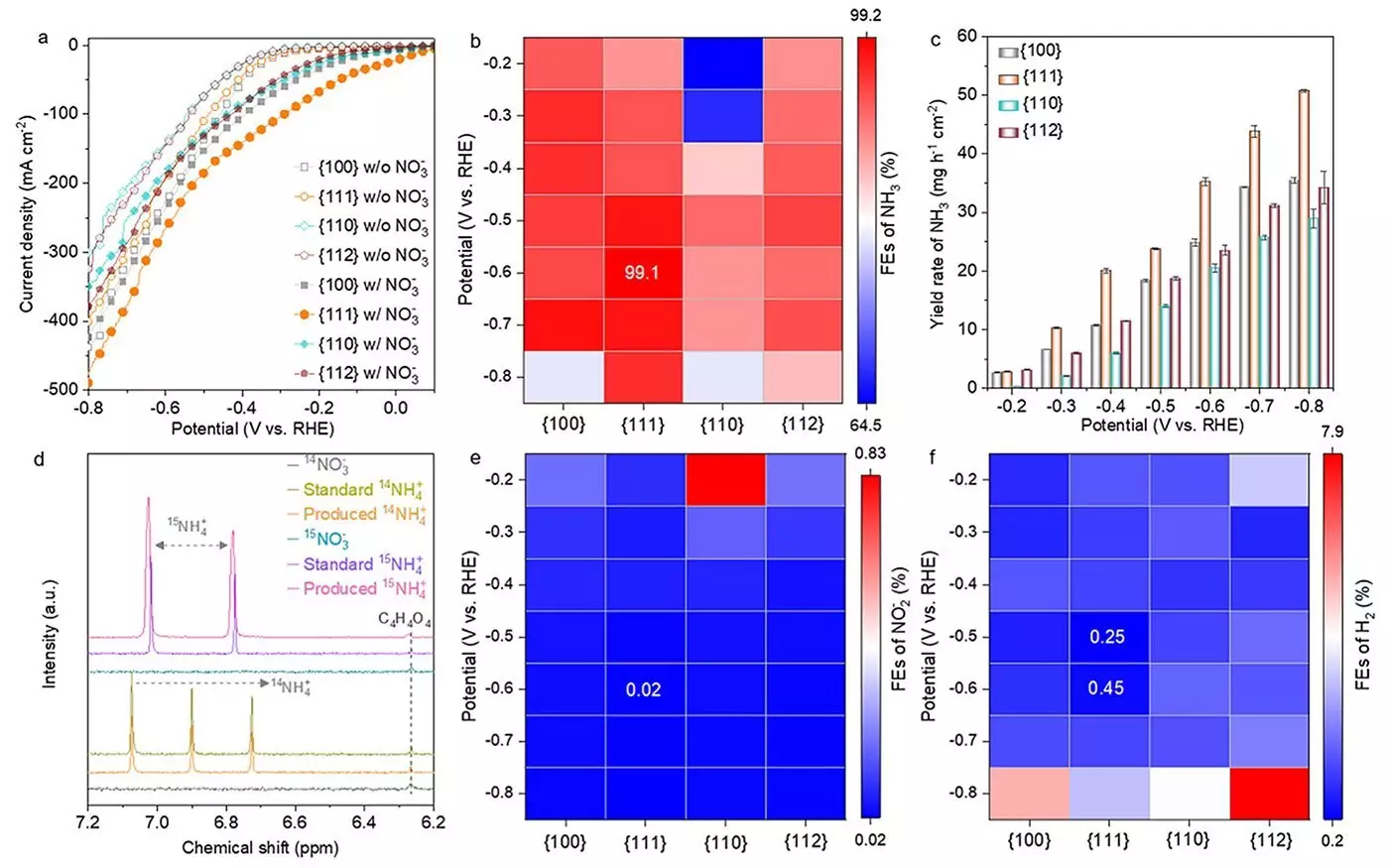
In their pursuit of optimal catalysts, the research team focused on various nanostructures of Co3O4, specifically assessing the impact of different crystallographic facets—namely {100}, {111}, {110}, and {112}. This meticulous approach allowed them to determine which facet offered the best performance in the ammonium production process. The findings were striking: the {111} facet of Co3O4 demonstrated superior catalytic capabilities, achieving a Faradaic efficiency of 99.1% and an ammonia yield rate of 35.2 mg h-1 cm-2. This unparalleled performance can be attributed to the unique properties of the {111} facet, which promotes the rapid formation of oxygen vacancies—crucial for increasing catalytic efficiency.
An intriguing aspect of this study was the discovery that the Co3O4 catalyst does not maintain a consistent structure throughout the reaction. Instead, it undergoes a complex transformation, starting from Co3O4, evolving through a mixture of phases including Co3O4−x-Ov/Co(OH)2, and ultimately stabilizing into Co(OH)2. This transition is particularly pronounced on the {111} facet and plays a key role in the catalyst’s enhanced performance. Understanding these structural dynamics is pivotal, as it provides insight into how modifications can lead to more effective catalytic systems. Such insights not only validate the performance of the {111} facet but also lay the groundwork for future catalyst design.
The implications of advancing catalysts for the electrochemical reduction of nitrates extend far beyond mere ammonia production. As a zero-carbon fuel, ammonia could revolutionize energy systems, providing a cleaner alternative in a world increasingly desperate for sustainable solutions. Moreover, by recycling nitrate waste through this process, the research contributes to environmental remediation efforts, showcasing the dual-benefit potential of this technology.
Professor Hao Li from the Advanced Institute for Materials Research emphasizes the critical nature of this research in shaping future catalyst development. “Our findings pave the way for creating more efficient, sustainable catalysts by optimizing their structures,” he noted. The goal is not just to enhance activity but also to ensure stability and selectivity in various reaction environments.
As we advance toward global targets for carbon neutrality, innovations like these in ammonia production are vital. The ability to convert hazardous waste into valuable products while eliminating the carbon costs of traditional methods positions researchers and industries alike on the frontline of environmental sustainability initiatives. Collectively, these breakthroughs could significantly alter industrial practices, reducing reliance on energy-intensive processes and aiding in the transition to cleaner, more sustainable energy solutions.
The recent advancements in the study of Co3O4 catalysts in the eNO3RR mark a critical juncture in the quest for sustainable ammonia production. By marrying the goals of agricultural fertilizer needs with energy transition strategies, researchers are not just solving current challenges; they are laying the foundation for a greener, more efficient future that balances human needs with ecological responsibilities.