In contemporary chemical manufacturing, the reliance on organic solvents for the synthesis of organic molecules poses a significant environmental challenge. A staggering 80% of waste generated from these processes is attributed to these toxic solvents, which often lead to improper disposal and ecological harm. Chemical reactions traditionally take place in liquid phases, as they facilitate effective substrate interactions; however, many essential substrates and catalysts are inherently sensitive to water, leading to side reactions that yield undesirable by-products. This precarious situation demands that synthetic chemists turn to hazardous organic solvents, which only compounds the dilemma facing the chemical industry today.
Researchers from the Indian Institute of Science (IISc) have made noteworthy advancements in addressing these pressing issues. Their novel approach involves the development of a surfactant derived from agricultural waste, specifically cashew nut shell liquid (CNSL). This sustainable and bio-based solution is tailored to enable micellar catalysis in aqueous environments, thus replacing the conventional reliance on organic solvents. The groundbreaking study was recently published in ACS Sustainable Chemistry & Engineering, revealing a transformative vision for chemical synthesis.
Pritesh Keshari, the primary author and a doctoral student at the Department of Inorganic and Physical Chemistry (IPC), underscores the significance of utilizing an eco-friendly alternative. With India ranking as the second-largest producer of cashew nuts globally, CNSL is both cost-effective and readily available, enabling a shift toward more sustainable practices in chemical synthesis.
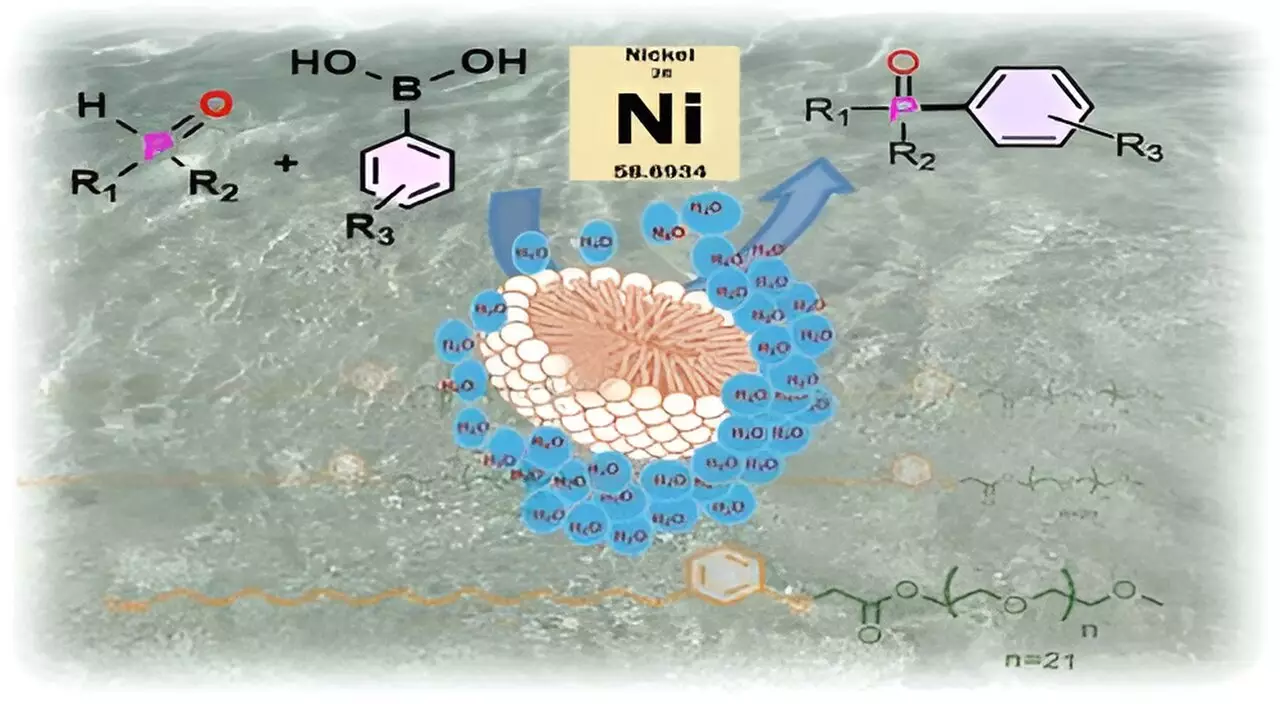
The surfactant devised by the research team, referred to as CNSL-1000-M, features a molecular structure composed of a hydrophobic component from cardanol in CNSL and a hydrophilic polymer, m-PEG. When introduced to water, these surfactant molecules spontaneously form micelles—small, spherical structures that protect sensitive substrates and catalysts from the detrimental effects of water.
The behavior of these micelles can be likened to a football floating on a body of water. Just as the football contains air and remains unaffected by the surrounding water as long as it does not leak, the micelle encapsulates its payload, keeping the substrate and reactive product shielded from direct interaction with the water. This unique property enables chemists to facilitate reactions in an environment that would typically be inhospitable for certain compounds.
One of the remarkable applications of the CNSL-1000-M surfactant is in the formation of carbon-phosphorus bonds, a pivotal step in the synthesis of various important compounds like the anticancer drug Brigatinib and organic light-emitting diodes (OLEDs). The results speak volumes; utilizing the new surfactant yielded an impressive 80% increase in product output in water compared to traditional organic solvents. Furthermore, it outperformed existing surfactants by delivering a 30% improvement in yield for reactions conducted in aqueous conditions.
This innovation is not merely theoretical; it opens avenues for significant cost reductions in the chemical manufacturing process. By allowing for the substitution of expensive precious metal catalysts, such as palladium, with more economical nickel complexes, and facilitating reactions at lower temperatures, this sustainable micellar catalysis could lead to profound changes in industry practices.
The research team is keen to collaborate with industrial partners to implement this sustainable technology into mainstream synthetic processes. They envision detailed studies aimed at refining micellar chemistry to harness its full potential in commercial applications. As the chemical industry grapples with growing environmental concerns and the quest for sustainability, the introduction of surfactants like CNSL-1000-M represents a pivotal step toward greener chemistry.
By prioritizing ecological stewardship without compromising on efficacy, this innovative work serves as a vital reminder of the possibilities that lie within the intersection of waste reduction, sustainability, and synthetic chemistry. As more research unfolds, the prospect of moving away from harmful organic solvents could lead to a new era of environmentally conscious chemical manufacturing, one that protects both our planet and our health.