In a remarkable advancement within the field of molecular chemistry, a dedicated team of researchers led by Professor Ingo Krossing at the University of Freiburg is redefining the landscape of oxidation potentials. With this innovative approach, scientists have demonstrated an unprecedented ability to enhance the oxidation capacity of positive ions, paving the way for novel applications in various scientific disciplines. The team’s findings, recently published in *Nature Communications*, reveal that utilizing specific solvent and anion combinations can significantly elevate the potential of positive ions in oxidation processes.
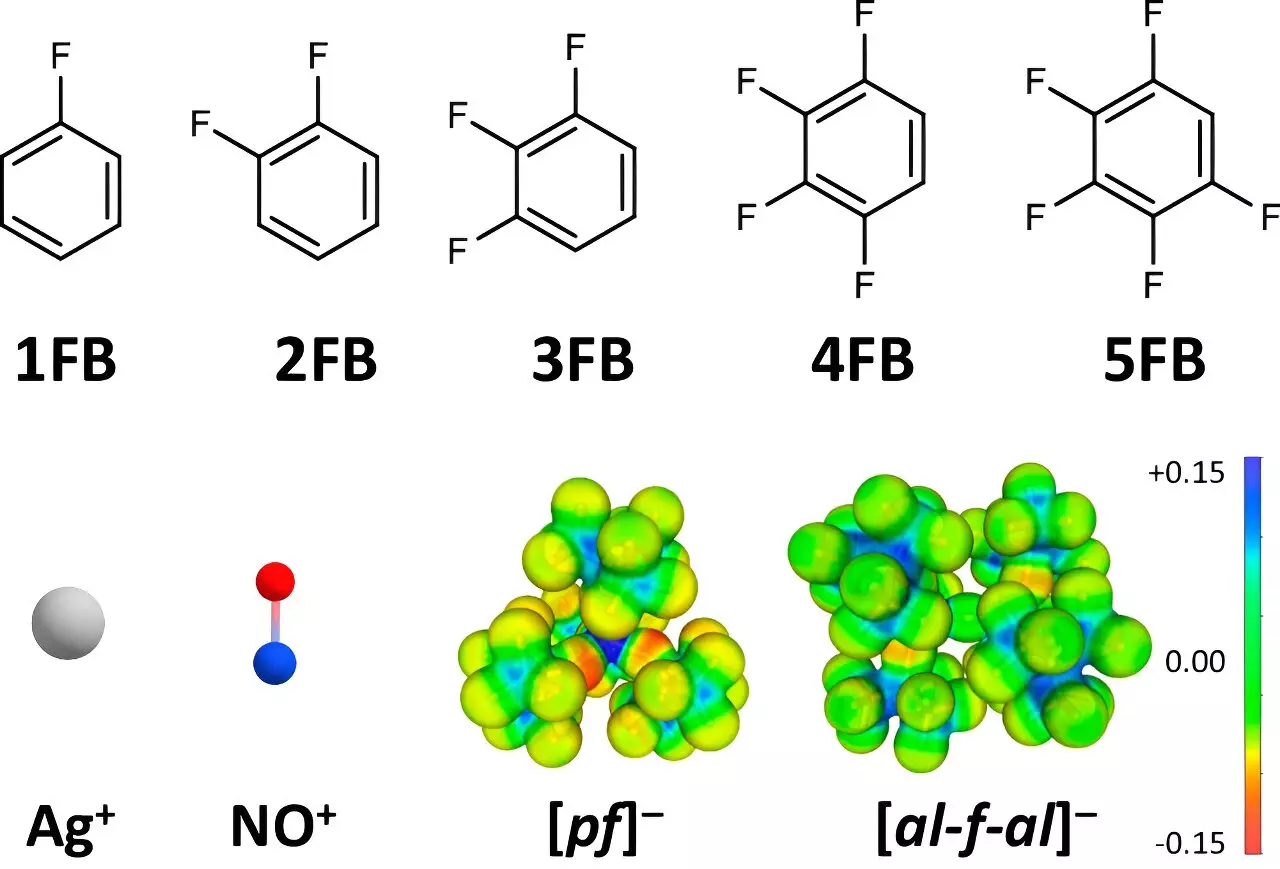
Understanding the dynamics of positive ions, such as Ag+ and NO+, as oxidizing agents has long posed challenges due to their interaction with the surrounding environment. Typically, these ions exhibit maximum oxidation potentials ranging from +0.65 to +1.0 V against the ferrocene (Fc+/0) standard. A notable limitation has been the reduction in oxidation potential caused by the strong interactions between these ions and conventional solvents or anions. This persistent issue has restricted the application of these ions in oxidation reactions, particularly when dealing with difficult-to-oxidize substrates or in advanced electrocatalytic processes.
The Freiburg research team adopted a unique approach to address this limitation by exploring the use of weakly coordinating anions (WCAs) and fluorinated benzene derivatives as solvents. In essence, the researchers identified that the degree of fluorination in aromatic compounds plays a crucial role in minimizing undesirable interactions. By shifting focus to solvents like disubstituted and tetrafluorinated benzene derivatives, the scientists unlocked higher dielectric constants compared to traditional solvents such as dichloromethane and acetone. This strategic selection allows for enhanced stability of positive ions, thereby facilitating improved oxidation potentials.
Collaborating with Dr. Johannes Hunger from the Max Planck Institute for Polymer Research, the team meticulously analyzed key dielectric properties of these fluorinated solvents. This collaboration was pivotal in understanding how the molecular environment affects ionic behavior. The findings revealed that as the aromatic compounds exhibited increasing fluorination, there was a corresponding increase in oxidation potential. Such insights are vital, not just for theoretical understanding but also for practical applications in the synthesis and manipulation of chemical reactions.
To study the interaction between these fluorinated solvents and positive ions, the team employed single-crystal X-ray diffraction techniques to elucidate solid-state structures. This method provided empirical data confirming that the interaction decreases with the degree of fluorination. Co-author Dr. Malte Sellin articulated, “The diminishing interaction facilitates almost undisturbed species, enabling a robust environment for oxidation.” The structural insights derived from these experiments validated the hypothesis that strategic solvent and ion selection is instrumental in enhancing oxidation potentials.
The implications of this research extend beyond academic interest. Enhanced oxidation capabilities could transform electrocatalytic processes, promoting sustainable chemical transformations and facilitating more efficient energy storage and conversion systems. As Professor Krossing noted, the potential for new elementary chemical studies is immense, suggesting that entirely new applications could be on the horizon.
The remarkable findings of Professor Krossing’s team mark a significant milestone in molecular and coordination chemistry. By systematically utilizing weakly coordinating anions and selecting appropriate solvents, they have opened the door to a plethora of new chemical reactions and applications. This work exemplifies a crucial intersection between fundamental chemistry and practical applications, setting the stage for future research and innovation in both oxidative processes and catalysis. This revolutionary approach not only enhances our understanding of oxidation potentials but also contributes significantly to the advancement of materials science and chemical engineering.