Polymers, the complex structures formed by long chains of repeating units known as monomers, are ubiquitous in various industries due to their versatile applications. However, their potential is often limited by the uniformity of the building blocks used in their construction. Recent research led by chemists at Scripps Research, in collaboration with institutions like Georgia Tech and the University of Pittsburgh, has revealed a groundbreaking method to create diverse monomers through a nickel-catalyzed reaction. This innovation could significantly enhance the functionality and environmental sustainability of polymers.
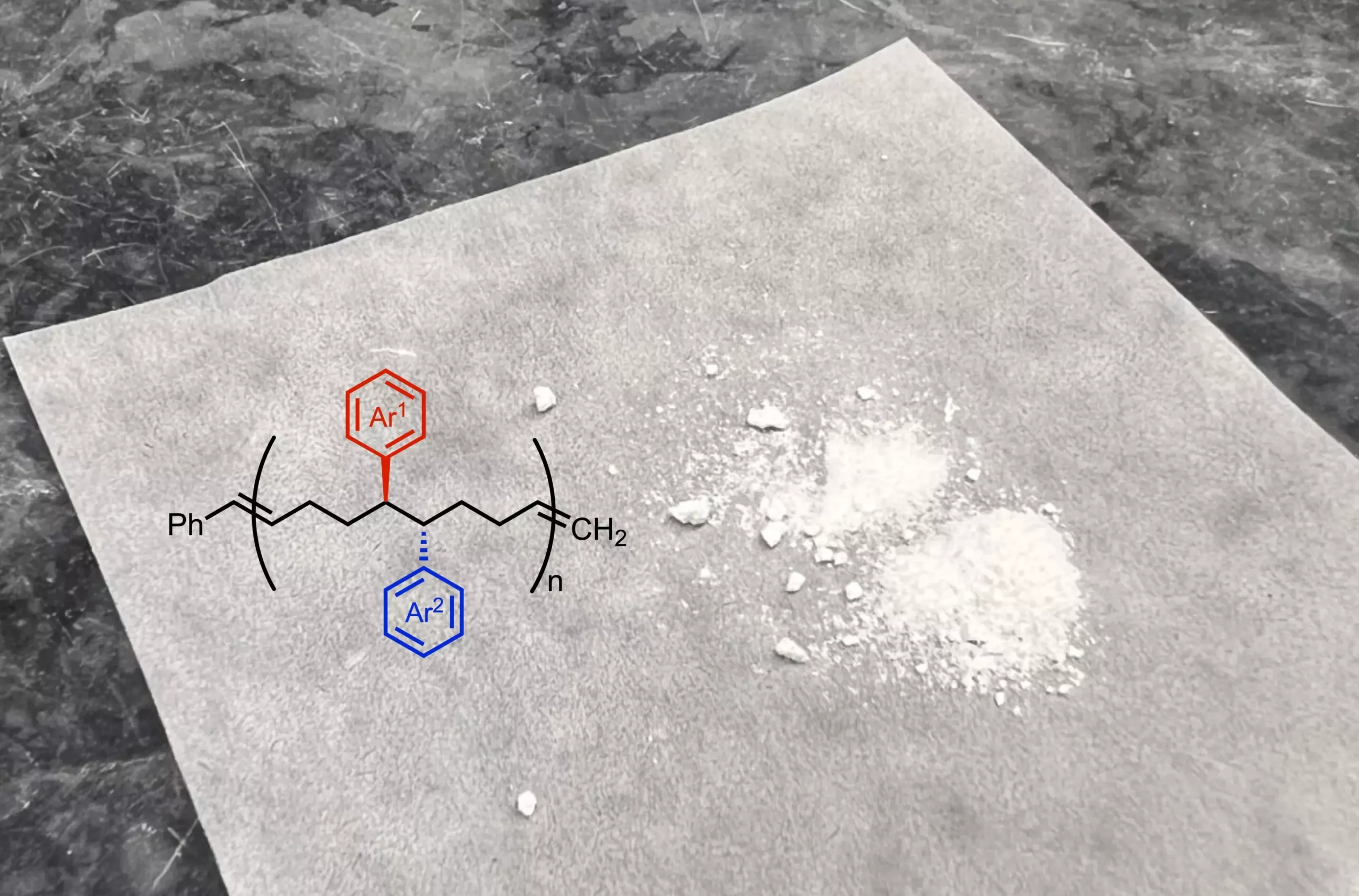
Conceptually, monomers can be seen as the individual train cars within a larger railway system, where each car contributes distinct properties to the entire train. Traditional polymer synthesis relies on a limited set of chemically similar monomers, which constrains their structural and functional diversity. The new research aims to disrupt this paradigm by enabling the synthesis of unique monomers through a controlled chemical process. By altering the functional groups on the monomers—similar to adding new features to each train car—scientists can create polymers with tailored characteristics suitable for specific applications, such as in drug delivery systems, energy storage solutions, and advanced microelectronics.
Nickel, a more abundant choice among metal catalysts, plays a pivotal role in this new approach to monomer synthesis. The Scripps Research team developed a novel reaction that modifies a starting molecule, adding two new functional groups that can significantly influence the properties of the resultant polymer. These functional groups act as tiny side chains, altering important traits of the polymer, from flexibility to solubility. This innovative approach not only expands the toolkit available for polymer synthesis but also opens doors to the creation of materials that possess a distinct set of physical and chemical properties compared to traditionally manufactured polymers.
The recent study, published in *Nature Synthesis*, reflects a collaborative effort among researchers dedicated to pushing the boundaries of polymer and material science. Under the direction of Dr. Keary Engle, the research team sought to determine if techniques typically used to generate small molecules could be effectively applied to the synthesis of larger polymeric structures. Lead co-authors, including Anne Ravn and Van Tran, contributed valuable insights during the development phase, testing the scalability of their nickel-catalyzed approach alongside polymerization techniques from their collaborators.
Dr. Ravn emphasizes the critical role of functional groups in polymer properties: “By modifying the chemistry of the initial building blocks, we can significantly impact the macromolecular structure.” Unlike typical commercial polymers that maintain a uniform structure, the new polymers exhibit closer functional group spacing, resulting in materials that boast enhanced elasticity and molecular interactions.
One of the striking implications of this research is its potential impact on sustainability in polymer production. Traditional methods often rely on rare and expensive metal catalysts, raising concerns regarding environmental safety and resource depletion. In stark contrast, the researchers argue that their nickel-catalyzed method could serve as a more sustainable alternative while also allowing further exploration into the degradation and recycling of polymers. Ravn emphasizes the importance of creating a system that enables the reuse of materials by breaking them down into their original building blocks.
Future research aims to examine how substituting various functional groups onto the newly formulated monomers might affect polymer properties further. This adaptability enables a greater exploration of the functionalities available to create innovative materials aimed at specific applications.
The advancements highlighted in this study signify an important milestone in polymer chemistry, paving the way for a new era of material science. By unlocking a pathway to create diverse monomers via sustainable practices, researchers are not only broadening the horizons for industrial applications but also addressing pressing environmental challenges. As the team continues its efforts to refine the methodology and explore novel functional groups, the potential for transformative changes in polymer technology remains extensive, positioning this area of research at the forefront of scientific innovation.
The evolution in monomer synthesis propelled by the Scripps Research team’s efforts exemplifies a promising intersection between chemistry, sustainability, and technological advancement, forging a new landscape for the creation and application of polymers.