Microplastics are an insidious pollution problem that infiltrates almost every aquatic ecosystem. Their presence raises significant environmental concerns, particularly regarding their fate in changing climatic conditions. Recent research published in *Environmental Science & Technology* provides a groundbreaking examination of how freezing and thawing cycles—common in many freshwater and marine systems—affect these minute plastic particles. The findings reveal that the structural integrity and behavior of microplastics can be altered by ice, influencing their environmental impact and distribution.
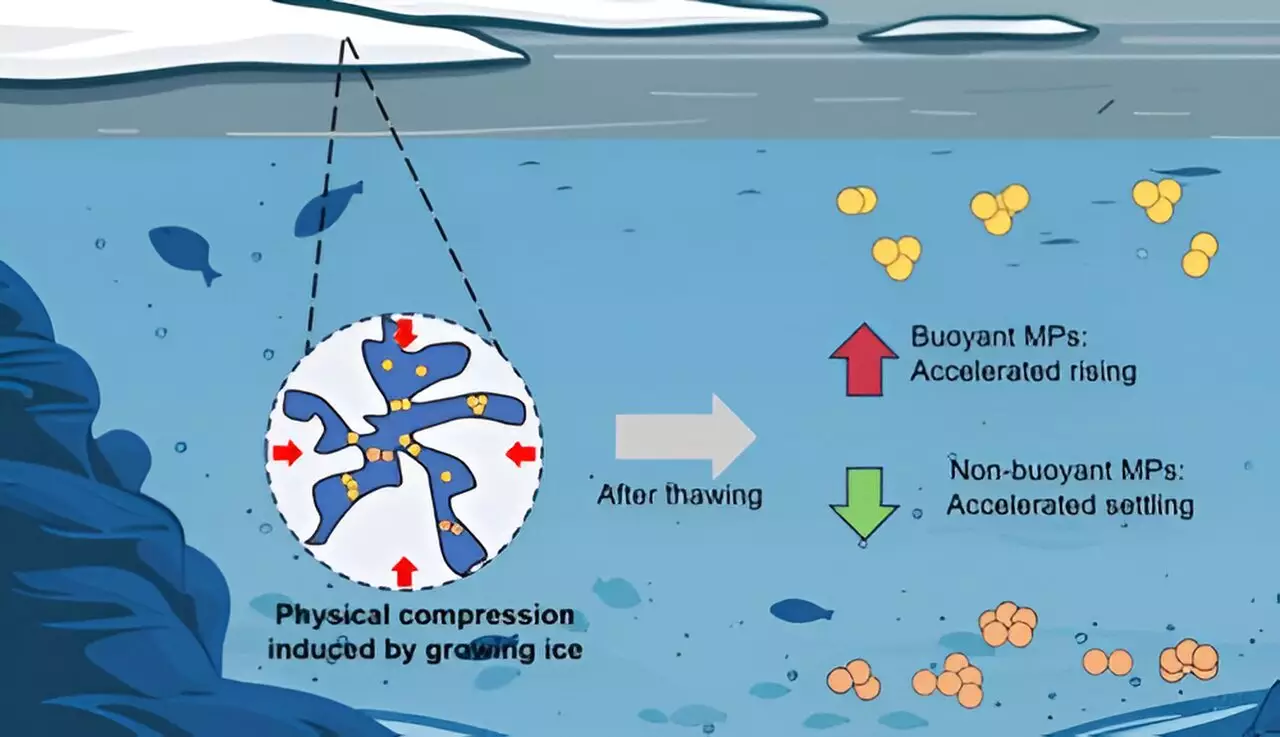
Traditional understanding suggests that microplastics simply persist in the environment; however, the new research introduces an important variable: ice. When water freezes, it can trap microplastic particles just beneath the surface, but this entrapment isn’t merely an inert storage mechanism. As the ice melts, the microplastics do not simply return to their original state; they undergo size transformations, impacting whether they rise to the surface, remain suspended, or sink to the depths.
The study assessed microplastic particles made from different polymers—specifically polyethylene (PE), polyurethane (PU), and polytetrafluoroethylene (PTFE)—to explore how each type reacted to freezing and thawing in both freshwater and saline environments. It turns out that these polymers exhibit distinct behaviors fundamentally determined by their density relative to water and their interactions with ice.
Effects of Polymer Nature on Microplastic Behavior
The researchers observed that the physical characteristics of the polymers played a central role in determining whether they would float, sink, or remain suspended after thawing. PE, being less dense than water, consistently floated. On the other hand, PU and PTFE—both denser than water—tended to sink. This difference is crucial, as it implies that the environmental impact of microplastics can vary significantly based on polymer types. Understanding this differential behavior can inform remediation efforts and risk assessments pertaining to microplastic pollution.
An interesting finding was the substantial increase in size of the PE particles (up to 46%) following freezing and thawing, compared to a mere 9% increase in PU particles. Such variability suggests that the hydrophobic nature of PE might contribute to particle clustering, while the hydrophilic attributes of PU would enable greater dispersion. This directly influences the possibility of microplastics accumulating in freshwater sediments and lake beds after the ice has melted.
Salinity and its Role in Microplastic Stability
Another critical aspect of the research involves salinity levels in the water samples. The team discovered that increasing salinity negated the size increases observed in freshwater. This observation highlights the complex environmental factors influencing microplastic behaviors. Higher salinity likely creates brine channels in the ice which prevent particles from being compressed into larger aggregates, thereby maintaining their individual states.
These insights suggest that coastal and estuarine environments—where salinity dynamics differ significantly from freshwater systems—may experience different levels of risk regarding microplastic accumulation and distribution.
While the initial findings provide a critical foundation for understanding microplastic behavior associated with ice, the researchers caution against overgeneralization. The short-duration laboratory freezing in this study contrasts with the prolonged freezing periods occurring in natural environments, which could yield different results. As climate change continues to impact these freezing cycles, ongoing research is essential for mapping out the long-term consequences of microplastic behavior.
Continued examination of how various environmental factors impact microplastic dynamics can significantly enhance our predictive capabilities regarding their ecological impacts. Policymakers can benefit from these findings in designing effective strategies aimed at mitigating microplastic pollution and preserving aquatic ecosystems that are increasingly vulnerable to anthropogenic pressures.
As the ice melts in our lakes and rivers, microplastics may emerge transformed—both in size and behavior—potentially altering their ecological fate forever. Understanding these changes is essential not only for addressing current pollution challenges but also for predicting future environmental health in aquatic ecosystems.