As the world increasingly shifts towards sustainable materials, polypropylene has emerged as a backbone of modern products. This versatile plastic is found in countless applications, from food containers to essential medical devices. The burgeoning demand for polypropylene pinpoints a critical need for propylene, its precursor chemical. Traditionally sourced from propane, propylene’s manufacturing process has remained energy-intensive and environmentally taxing. As a solution, recent research conducted by notable scientists at the U.S. Department of Energy’s Argonne National Laboratory and Ames National Laboratory offers a transformative approach to propylene production that could significantly alter the landscape of industrial catalysis.
Innovation in Catalysis: A Groundbreaking Method
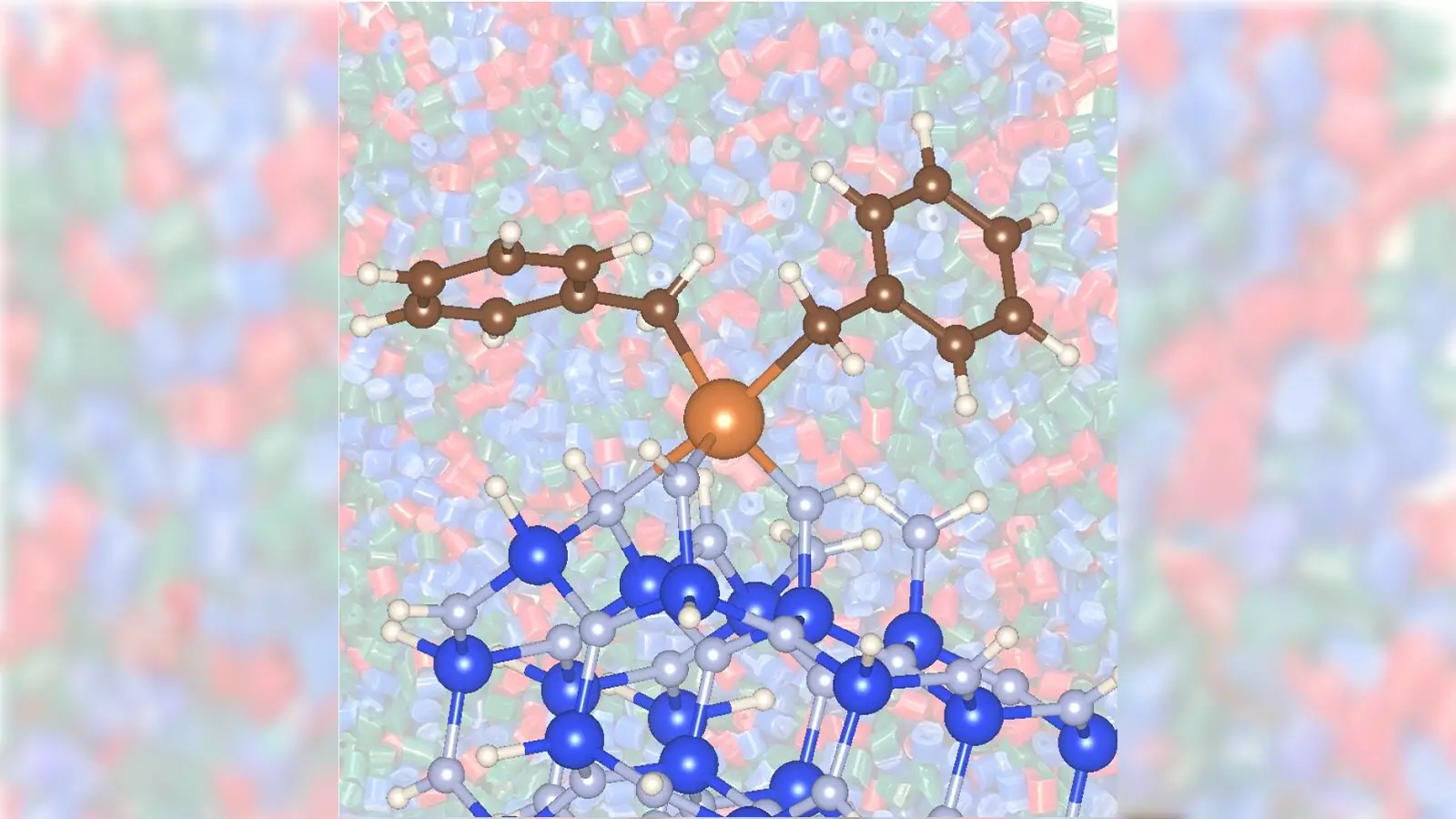
The existing methodology for converting propane into propylene relies heavily on metal catalysts such as chromium or platinum, often requiring high operational temperatures and significant energy expenditure. The newly reported method, however, employs a combination of zirconium with silicon nitride, leading to a more efficient and less toxic process. Not only does this innovative approach promise faster conversion rates, but it also curtails the energy demands typically associated with propylene production.
The research, published in the Journal of the American Chemical Society, highlights the performance of zirconium on silicon nitride as a remarkable advancement over traditional catalysts. The findings suggest that this combination enables the catalytic conversion of propane at a substantially reduced temperature of 842 degrees Fahrenheit, compared to the standard 1,022 degrees Fahrenheit typically necessary when using conventional methods. Such a dramatic drop not only saves energy but also translates to lower carbon dioxide emissions, addressing a crucial factor in the ongoing battle against climate change.
Reducing Environmental Impact
At a time when carbon dioxide emissions account for nearly 80% of greenhouse gas outputs in the United States, the implications of this research cannot be overstated. The ability to reduce the temperature of the catalytic process directly correlates to diminished CO2 emissions. Furthermore, the utilization of zirconium over traditionally precious metals like platinum represents a cost-effective alternative that could facilitate broader adoption of this eco-friendly approach across various industries.
Research lead David Kaphan pointed out that this novel methodology also opens doors to the use of other low-cost metals in catalytic reactions, potentially broadening the availability and affordability of sustainable production techniques. These pioneering findings not only extend to propane to propylene conversion but provide a roadmap for future exploration of alternative reactions using transitional metals, all while keeping environmental concerns in view.
Not Just Another Catalyst
The unique properties of silicon nitride as a catalyst support material are pivotal to the success of this new method. Traditionally used oxide supports have limitations that the silicon nitride technology deftly sidesteps, providing an enhanced chemical reaction dynamic that promotes greater catalysis efficiency. It’s intriguing to note that silicon nitride facilitates reactions not just differently but potentially more effectively than their conventional counterparts.
The method shows increased reactivity and production efficiency due to the specific surface interactions afforded by silicon nitride. This innovative approach challenges the established methodologies, demanding that researchers reconsider the materials they’re using to enhance solution dynamics in chemical processes.
A Collaborative Success Story
This research didn’t materialize in isolation; it is the product of diligent teamwork among leading scientists from Argonne and Ames National Laboratories. With a blend of materials science expertise, they pioneered groundbreaking techniques such as X-ray absorption spectroscopy and dynamic nuclear polarization-enhanced nuclear magnetic resonance. The collaborative effort underscores the necessity of multidisciplinary approaches in tackling modern scientific challenges.
Frédéric Perras, a scientist from Ames, expressed excitement about the largely unknown interactions on the silicon nitride surface that were uncovered during the research. This exploration of material properties demonstrates that there are still vast unknowns in catalysis that could lead to further advancements in sustainable technologies.
The collective insights of this team illuminate just how interconnected various scientific disciplines are, reinforcing the idea that innovation thrives on collaboration. As Delferro remarked, the success of this work is a testament to the power of diverse expertise converging towards a common goal.
The Path Ahead
As industries strive for greater sustainability, the potential applications of this innovative catalytic method extend far beyond just propylene production. It paves the way for greener practices across various chemical manufacturing sectors, presenting a forward-thinking model for how we can harness natural resources more efficiently. The implications of this research reverberate through not just chemical engineering practices, but also through environmental policy discussions, as societies become increasingly focused on reducing their carbon footprint and transitioning towards a more sustainable future.