In a remarkable stride towards sustainability, a research team led by Professor Jaeheung Cho at UNIST has unveiled a cutting-edge catalyst that emulates the natural enzymatic processes utilized for breaking down harmful hydrocarbons. This breakthrough marks a significant leap for environmental chemistry, promising not only to improve pollution mitigation but also to push the boundaries of energy efficiency in chemical reactions. The findings, published in the esteemed Journal of the American Chemical Society, highlights how this innovative discovery could redefine how industries approach hydrocarbon degradation and sustainable practices.
The Science Behind the Catalyst
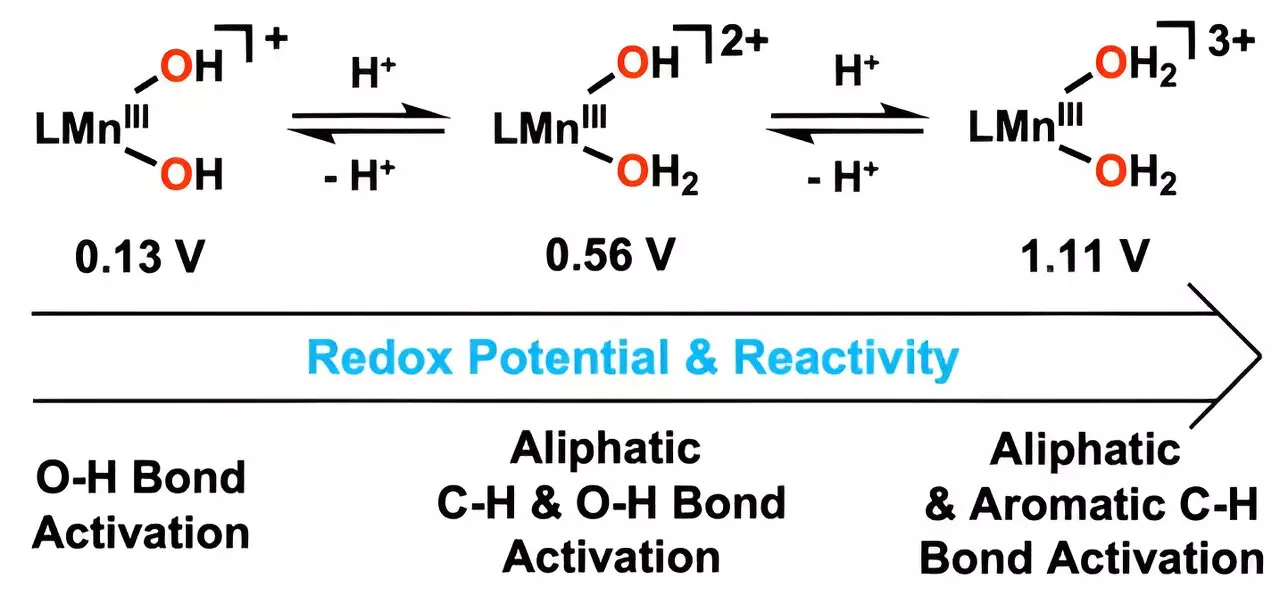
At the core of this advancement is the catalyst’s ability to mimic metalloenzymes, which are naturally occurring enzymes rich in metals known for their catalytic prowess. What sets this research apart is its use of manganese in a refined state, enhanced by the strategic addition of hydrogen ions. By effectively modifying a hydroxyl ligand to include metal-bound water molecules, the new catalyst can engage in the oxidation of carbon-hydrogen (C–H) bonds at notably lower temperatures compared to traditional methods. This innovation is not merely academic; it translates to substantial energy savings and a reduction in by-products that typically accompany chemical reactions.
Process Efficiency and Environmental Impact
One of the standout aspects of this catalyst is its ability to decompose particularly resilient substances, such as anthracene, which is notorious for its strong C–H bonds. Through its application, this new catalyst demonstrates significant efficacy in oxidizing harmful hydrocarbons, including those that are water-insoluble and chemically stable. The downstream benefits of this technology are profound: reduced toxicity levels in industrial processes, less reliance on harsh chemical agents, and a streamlined pathway towards achieving cleaner emissions.
Professor Cho’s assertion that this is the first successful instance of a manganese(III) complex combining with water molecules to react with aromatic hydrocarbons effectively expands the horizons of catalytic science. The increased reduction potential of manganese, as facilitated by this innovative approach, distinguishes this catalyst, enhancing its capacity to engage with oxygen-hydrogen bonds more efficiently.
Future Implications for Industrial Chemistry
The implications of this research extend well beyond academic circles. As industries grapple with increasing regulatory pressures to reduce their environmental footprint, the development of advanced catalytic systems like this one could pivotally shift operational protocols. By enabling processes that were once deemed impractical due to energy costs and chemical reactivity, this new catalyst may incite a wave of innovation in how chemicals are synthesized, promoting a more sustainable industrial ecosystem.
In a landscape where ecological sustainability and efficiency are paramount, breakthroughs like this underscore a critical shift towards greener alternatives in chemistry. This catalyst not only embodies the potential to address pressing environmental challenges but also highlights the ongoing need for interdisciplinary collaboration in fostering sustainable development. As we embrace these advancements, the future of clean chemical processes appears promising, fortified by science that harmonizes with nature’s own methods.