The relentless pursuit of sustainable energy solutions has led chemists and material scientists to innovate towards converting waste products into high-value materials. The urgency for eco-friendly alternatives has never been more pronounced, with climate change and resource depletion urging the scientific community to think outside the box. A recent collaborative study published in Nature Catalysis has shed light on a groundbreaking method that utilizes electricity to efficiently convert carbon dioxide—a significant greenhouse gas—into methanol, a promising liquid fuel. This innovative approach not only aims to address waste management issues but also positions methanol as a viable transitional fuel in our move towards a sustainable energy future.
The Mechanism Behind the Innovation
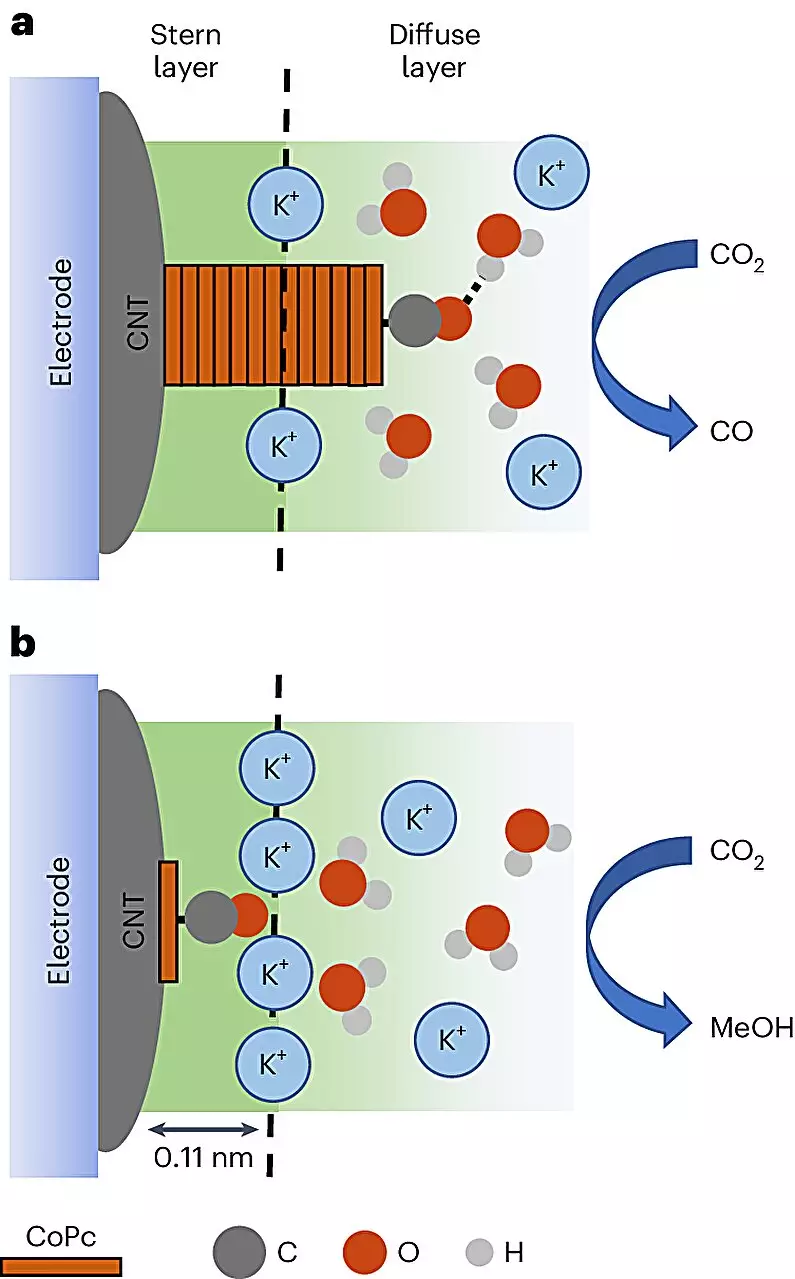
The researchers employed cobalt phthalocyanine (CoPc) molecules dispersed on carbon nanotubes, noted for their unique electrical properties. This combination created a platform where an electrolyte solution could be introduced. Running an electric current through this setup enabled the CoPc to convert carbon dioxide into methanol or carbon monoxide, with the reaction’s direction heavily reliant on the immediate environmental conditions surrounding the carbon dioxide molecules. This nuanced methodology illustrates a significant shift; researchers have uncovered that they can influence the efficiency of methanol production—enhancing it by as much as eight times—by optimizing the distribution of the catalyst on the carbon nanotube surface.
This revelation speaks to a larger trend in scientific inquiry: precision in the reaction environment is vital for producing desired outcomes. Robert Baker, a prominent figure in this study and a professor at The Ohio State University, emphasizes the compelling potential of transforming carbon dioxide into highly energy-dense fuels like methanol. This not only secures a pathway out of environmental crises but also redefines waste transformation, marking a crucial step toward energy self-sufficiency.
The Role of Advanced Spectroscopy in Chemical Insights
One of the pivotal factors that sets this study apart from past efforts is the ability to visualize and analyze chemical reactions in real time. Traditional empirical methods often yield satisfactory results, but without a comprehensive understanding of molecular behavior, the optimization of such reactions remains largely speculative. Through the use of advanced vibrational spectroscopy techniques, the research team achieved unprecedented clarity in observing how the CoPc molecules respond under varying conditions.
Lead author Quansong Zhu highlighted how these vibrational signatures allowed his team to ascertain the environmental factors influencing reaction pathways. Such nuanced insights enable chemists to make informed decisions regarding catalyst selection and reaction conditions—ultimately refining processes to maximize methanol yields efficiently. By peeling back layers of complexity surrounding these reactions, the research has paved the way for a stronger grasp of molecular interactions, a critical factor in advancing catalytic technology.
The Promise of Methanol: More Than Just a Fuel
Methanol’s significance extends beyond its role as an alternative fuel. The compound boasts a high energy density, making it an ideal candidate for a variety of applications including heating and power generation. When synthesized from renewable electricity, methanol can serve as an eco-friendly substitute for fossil fuels, applicable in different sectors ranging from transportation to industrial processes. Importantly, as climate policy pushes for lower emissions, methanol’s capacity to function as a transitional fuel further emboldens its role in the energy landscape.
In addition to its practical applications, the ongoing study into the interactions of CoPc molecules with charged particles known as cations hints at a plethora of untapped opportunities in chemical research. The quest to understand the influence of these particles on methanol production may unlock new avenues for catalysis and energy material science.
A Roadmap for Future Research and Implications
While the findings from this study mark a significant milestone within the realm of chemical synthesis, much work remains. Continued exploration into the impacts of environmental parameters on catalysis will only enhance our understanding and application of these systems. The collaboration between chemists and material scientists opens the door to interdisciplinary research that can lead to even more innovative energy solutions.
As researchers like Baker underscore the importance of comprehending molecular-level interactions, we stand at a unique juncture in scientific advancement. The potential to create a more sustainable world through such groundbreaking research fuels excitement and optimism, as industries and policymakers begin to envision a future empowered by renewable energy sources. In harnessing the powers of technology and nature, we are not only solving waste problems but enriching the landscape of chemical processes—solving the riddle of efficiency while lighting the way for a cleaner, brighter tomorrow.