In an era where environmental concerns loom large, the problem of micropollutants—minute pollutants like pesticides and trace chemicals—has become a focal point for researchers and policymakers alike. These contaminants, often overlooked due to their small size, pose a significant threat to ecological systems and human health. Conventional methods of water treatment are often ineffective at tackling these stubborn pollutants, necessitating innovative approaches that can purify our water resources more efficiently. Herein lies the potential of photocatalysis, a process that harnesses the power of light to generate chemical reactions capable of breaking down harmful substances.
The Photocatalytic Breakthrough
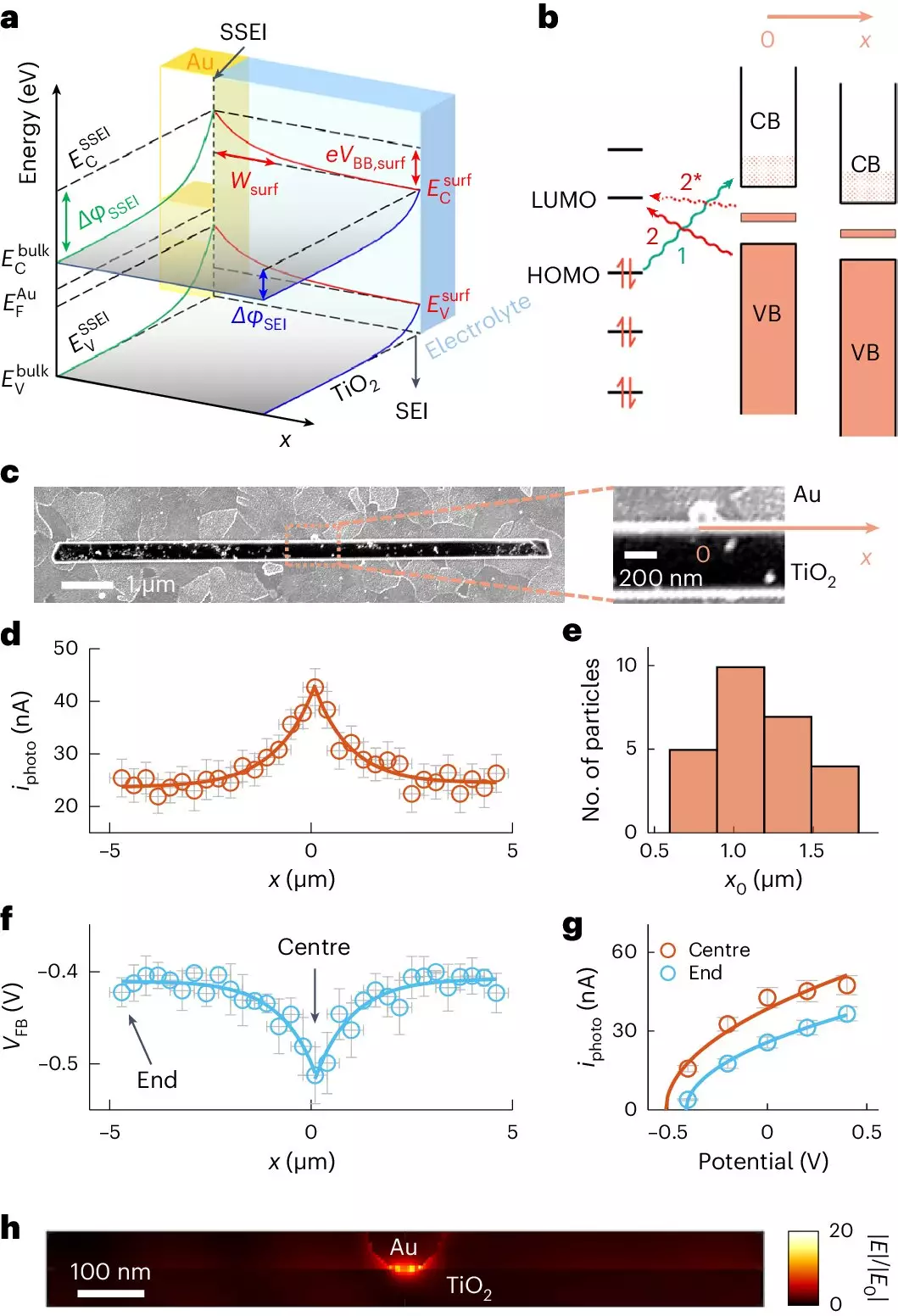
At the forefront of this innovation is a recent study from a team at Cornell University, which has unveiled promising advancements in photocatalytic technology using titanium dioxide (TiO2) enhanced with gold nanoparticles. Photocatalysis traditionally utilizes energy from sunlight to drive chemical reactions, and this research bolsters the efficacy of such processes. By employing state-of-the-art optical imaging techniques, the researchers were able to deeply investigate the adsorption dynamics of micropollutants onto semiconductor surfaces, specifically TiO2, mixed with gold.
A striking aspect of this study is the considerable scope of adsorption enhancement. The researchers discovered that gold nanoparticles, though diminutive themselves, vastly increase the area over which micropollutants cling to the TiO2 surface—distances extending more than a micrometer from the gold contact points. This extraordinary finding not only highlights the effectiveness of gold as a co-catalyst but also reshapes our understanding of how semiconductor materials interact with pollutants.
Innovative Imaging Techniques
Central to the success of this study is the development of a groundbreaking imaging technique known as adCOMPEITS, which stands for Adsorption-based COMPetition Enabled Imaging Technique with Super-resolution. By employing a fluorescent probe that competes with nonfluorescent micropollutants for surface adhesion, researchers can create an unprecedented map of adsorption across the TiO2 surface. The transformational quality of this method lies in its ability to visualize how pollutants interact at the nanoscale, revealing competitive adsorption dynamics that were previously hidden from view.
Ming Zhao, the paper’s lead author, eloquently articulates the implications of their findings: “The gold nanoparticle is only 100 nanometers in size. We discovered that it enhances adsorption microns away from where the particle is.” This clarity sheds light on the long-distance influence of gold nanoparticles, a phenomenon that challenges long-held assumptions regarding the localized nature of chemical interactions on semiconductor surfaces.
The Physics Behind the Phenomenon
One of the most compelling takeaways from this research is the concept of “surface band bending.” By altering the electronic properties of TiO2, gold particles induce changes that facilitate adsorption over vast distances. Essentially, the gold nanoparticles create an electrochemical environment conducive to attracting micropollutants even when they are not immediately adjacent. This resurfacing has profound implications, from enhancing the efficiency of photocatalysis to potentially transforming other fields like sensing technologies and solar energy conversion.
Such advancements are crucial for the future of environmental remediation and renewable energy, particularly as efficiency in solar-to-chemical conversions has historically been a limiting factor in photocatalytic applications.
Broad Applications and Future Implications
Beyond merely addressing micropollutants, the implications of this research stretch far and wide. As Professor Peng Chen noted, the applications of this long-range enhancement can extend into various domains, such as sensor technology and dye-sensitized solar cells. If these findings are effectively translated into real-world applications, we could witness a paradigm shift in how pollutants are managed, paving the way for cleaner water and a more sustainable energy landscape.
This exciting research signifies a leap forward in our approach to addressing environmental contaminants. By creatively leveraging the unique properties of nanoparticles in photocatalytic systems, we are forging a path not only toward more effective methods of pollution removal but toward a greener, more resilient future. As this field continues to evolve, it leaves us hopeful about the integration of innovative technologies that could safeguard our planet for generations to come.