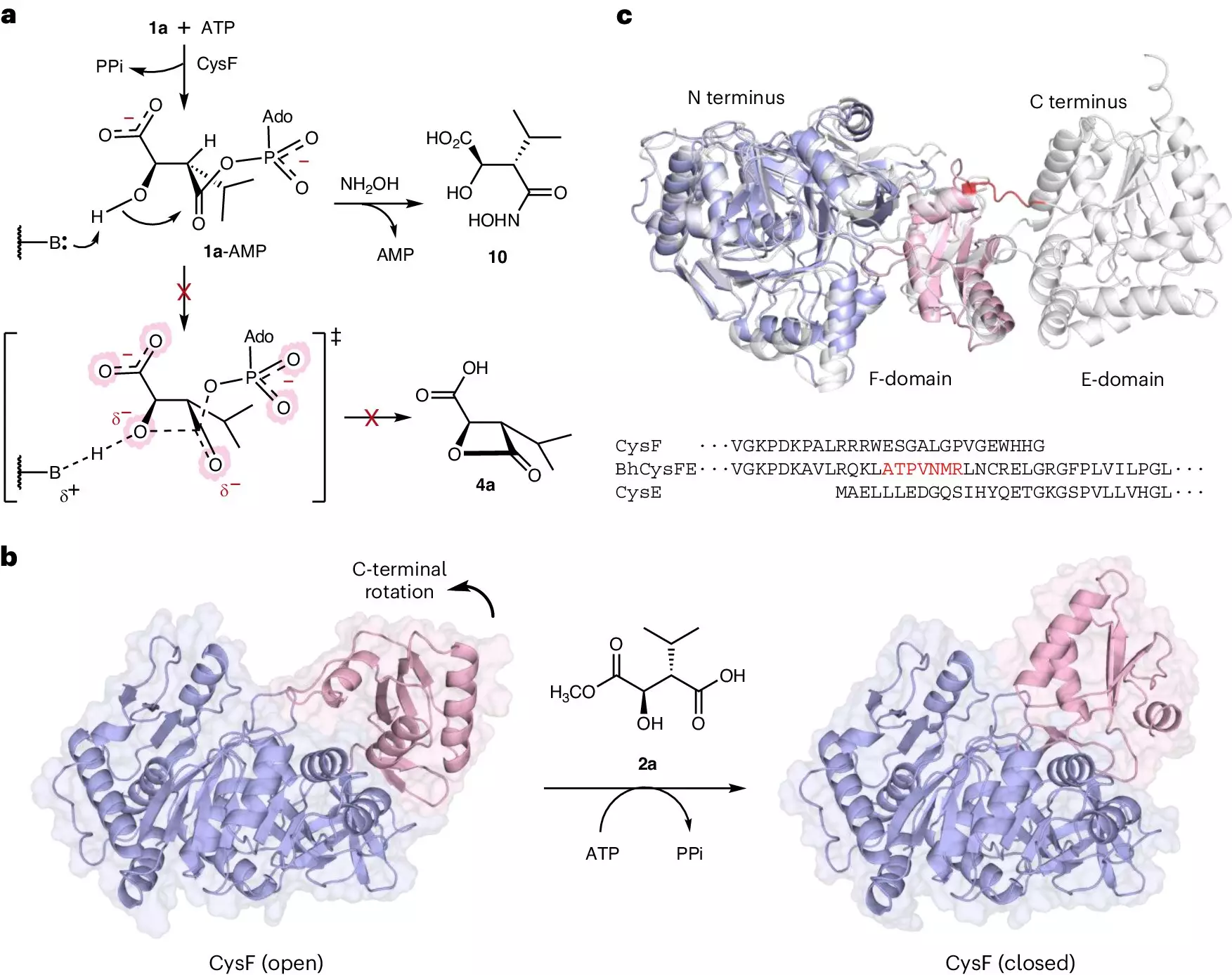
In the realm of pharmaceuticals, peptides are gaining recognition as crucial players in the battle against life-threatening diseases. Composed of small chains of amino acids, peptides serve not only as the building blocks of proteins but also as pivotal components in a variety of therapeutic applications ranging from cancer treatments to vaccines. The role they play in our health is paramount, yet their production has remained a complex and often environmentally detrimental process—until now. Researchers at the University of Manchester have made a groundbreaking discovery that could significantly change how peptides are created, presenting a more streamlined and eco-friendly method that promises to enhance their availability in clinical settings.
The Environmental Cost of Conventional Peptide Synthesis
Traditional methods of synthesizing peptides typically rely on intricate chemical processes that require numerous steps, often filling laboratories with a cocktail of hazardous waste. With a reliance on toxic reagents and volatile organic solvents, these outdated procedures not only hinder efficiency but also pose a severe risk to environmental health. The high costs associated with such labor-intensive methods create barriers to scaling production, limiting the availability of vital peptide-based medicines. These challenges have marred the potential of peptides to meet the urgent healthcare demands of the modern world. However, the new advancements by Manchester’s scientists herald a revolution in how we can overcome these obstacles.
A Breakthrough in Ligase Enzyme Technology
At the heart of this innovation lies a new family of ligase enzymes, which function as molecular “glues” that bond small peptides together more effectively. This remarkable technology has the potential to transform the landscape of peptide production. As highlighted by Professor Jason Micklefield, this technique allows for the production of peptides with promising anti-cancer properties in a single, streamlined process that ensures an impressive yield. Gone are the days of lengthy methods involving multiple steps—now, a combination of ligases can be used in a cascade reaction to generate various peptides rapidly while maintaining high efficiency.
The implications of this development are profound, particularly in oncology, where rapid and effective drug development is crucial. If this ligase-enabled approach becomes standard practice, it could reduce the time required for manufacturing treatments, ultimately translating into quicker access to life-saving medications for patients worldwide.
Decoding Nature’s Blueprint
The University of Manchester team’s research not only introduces new enzymes but also establishes a roadmap for future peptide synthesis. Through meticulous analysis of bacterial ligases that naturally construct peptides, the researchers have identified a multitude of related ligases that could further enhance the scope of peptide applications. Dr. Guangcai Xu, a key member of the team, underscores the versatility of the discovered ligases, stating their potential to construct longer peptides for an even broader range of therapeutic uses.
By tapping into the biological processes observed in simple organisms, this breakthrough shifts the paradigm from a purely synthetic approach to a more biological and sustainable model. This could mark a significant turning point, with the scientific community increasingly favoring environmentally-friendly methods that align with modern sustainability goals in pharmaceutical manufacturing.
Building a Collaborative Future
As the Manchester team embarks on optimizing these newly discovered ligases for commercial applications, they are also forging strategic partnerships with leading pharmaceutical companies. Such collaborations are essential to facilitate the transition from laboratory findings to real-world applications, ensuring that the benefits of these advancements can be realized on a larger scale.
The journey ahead is promising, with the potential to not just change peptide production processes but to lay down a marker for how future biotechnology innovations could unfold. The intersection of sustainability, efficiency, and therapeutic potential could take a significant leap forward, addressing urgent needs in healthcare while reigning in the harmful legacy of traditional chemical methodologies.
This remarkable study from the University of Manchester exemplifies the innovative spirit of science, offering a hopeful outlook on the future of peptide-based medicines. With each step forward, we edge closer to a world where essential medical therapies are not only more available but also produced in ways that nourish our planet rather than depleting it.