Ice is an everyday phenomenon that most people take for granted, yet its interaction with liquid water remains a fascinating and complex scientific puzzle. Ice frequently exists in the presence of liquid, shaping everything from our experiences on snowy slopes to the delightful sensation of a cold ice cream treat. A recent breakthrough from researchers at Kobe University and the Institute for Molecular Science shines light on the intricate interplay at the boundary where ice and liquid coexist. This study not only enhances our understanding of ice’s behavior but also opens new avenues for future research.
The Importance of the Ice-Liquid Interface
The interface between ice and water is essential for a multitude of natural processes. When we think of ice, we often picture it as a solid entity, yet at its surface, a layer of liquid is ever-present, subtly influencing its properties. Snowflakes crystallize, ice skates glide, and ice cream delights—all these phenomena are governed by the microscopic interactions between ice and its liquid counterpart. Previous challenges in observing these interactions have stalled advancements, as the rapid transition between ice and water makes direct observation nearly impossible. Given this context, the Kobe University team’s work marks a significant advancement in visualizing the enigmatic nature of ice under different conditions.
A Groundbreaking Approach Using Antifreeze
The researchers, led by Onishi Hiroshi, employed an innovative approach to study ice by immersing it in antifreeze liquids at temperatures below the freezing point. This technique prevents melting and allows for stable observation of the ice-liquid interface. The meticulous process required overcoming hurdles related to cooling and stability to ensure the atomic force microscope—a high-resolution measuring tool—could function effectively at sub-zero temperatures. This kind of innovative thinking is critical in scientific inquiry, often leading to breakthroughs that challenge our existing notions.
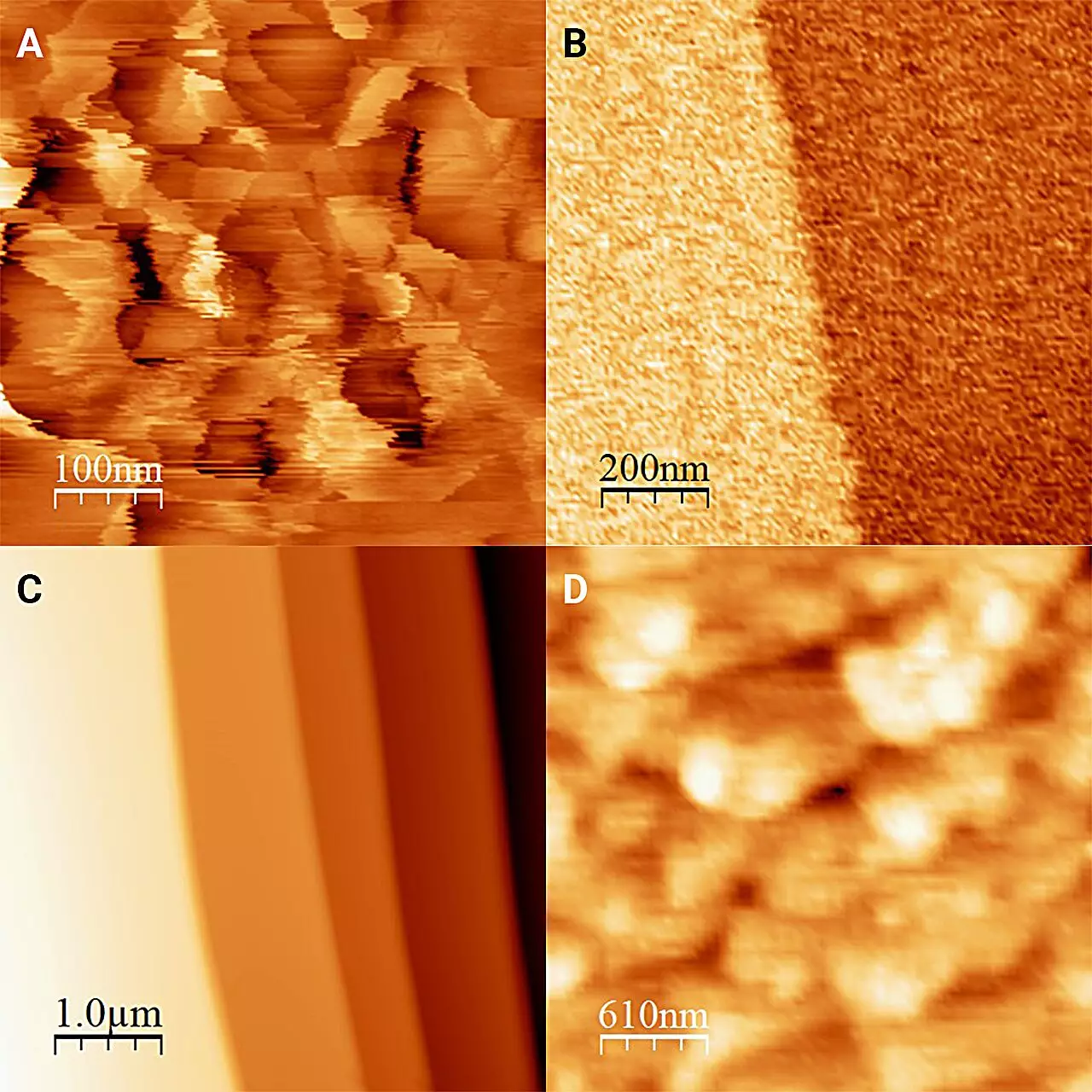
Revealing Surprising Findings
The results published in The Journal of Chemical Physics revealed unexpected characteristics of ice when in contact with antifreeze. While ice exposed to air is covered in “frost pillars” that measure approximately 20 nanometers in height, it was discovered that ice in antifreeze presents a remarkably flat surface, with only minor variations in height corresponding to molecular layers. This ambitious research suggests that partial dissolution and recrystallization occurs at the interface, leading to this smooth topology, which deviates significantly from conventional ice surfaces formed in less controlled settings.
Furthermore, the implications of using various alcohols highlight the nuanced chemistry involved at this interface. Although the alcohols tested share similar properties, each liquid produces distinct variations in the ice’s behavior and appearance. This variance points to the profound importance of studying individual interfaces rather than relying solely on historical assumptions about ice in contact with water.
Revising Ice’s Hardness
One particularly interesting aspect of the study was the revelation concerning the hardness of the ice surface. Traditional assessments have often led to underestimations of ice hardness, but the team’s findings indicate that ice surrounded by 1-octanol is significantly harder than previously evaluated. This new understanding could have far-reaching implications across multiple fields, from glaciology to materials science, as researchers begin to reassess the physical properties of ice under various conditions.
Future Directions in Ice Research
The team’s findings set the stage for even more intricate explorations of the ice-liquid interface. Onishi and his team aim to enhance the resolution of their research methods to the level of single water molecules, promising exciting possibilities in understanding the molecular dynamics that govern these interactions. Expanding upon their work, they express a desire to explore methodologies beyond atomic force microscopy, suggesting a multifaceted approach to future investigation.
In the realm of scientific inquiry, the intersection between ice and liquid offers endless possibilities. As this research unfolds, it not only bolsters our fundamental knowledge of materials but also inspires curiosity about the nature of ice itself—an everyday element that holds more secrets than we might ever realize. The next chapter in ice research beckons, and the scientific community waits with bated breath for more revelations from the frosty frontier.