After nearly 150 years since gallium was first identified by French chemist Paul-Émile Lecoq de Boisbaudran, a team of researchers at the University of Auckland has ushered in a new era of understanding of this fascinating metal. In a remarkable revelation, they have identified previously overlooked characteristics of gallium’s atomic behavior. This breakthrough is pivotal not only for chemistry but also for advancing fields like nanotechnology, where understanding the properties of materials at a microscopic level is crucial.
Gallium is renowned for its curious property of melting at a relatively low temperature, which allows a gallium spoon to dissolve if placed in a warm beverage. This physical anomaly perplexingly contrasts with traditional metal behaviors and has placed gallium in the spotlight for its unique applications in semiconductors and other technologies.
The Atomic Dance: Dimers and Covalent Bonds
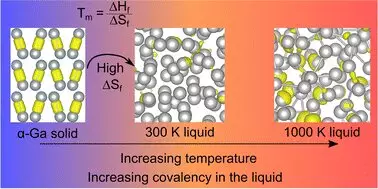
What sets gallium apart from most conventional metals is its existence as ‘dimers,’ which refers to pairs of gallium atoms that bind together. The phenomenon is indicative of its less dense solid state, a stark contrast to its liquid form — much like how ice manages to float on water. This duality in density is not merely a curiosity; it offers potential insights into the atomic forces that govern gallium’s behaviors.
Additionally, the presence of covalent bonds—where atoms share electrons—further complicates its classification as a metal. In an unexpected twist, researchers have demonstrated that while these covalent bonds vanish upon melting, they intriguingly re-emerge at elevated temperatures. This newfound understanding challenges long-standing assumptions about gallium’s melting point and necessitates a reevaluation of its physical properties.
From Theory to Practice: Implications of New Findings
The study, aptly titled “Resolving Decades of Debate: The Surprising Role of High-Temperature Covalency in the Structure of Liquid Gallium,” was published in the journal Materials Horizons. Professor Nicola Gaston, a prominent voice in this research, pointed out that the conventional wisdom held for over thirty years regarding the structure of liquid gallium might no longer hold true. Their fresh perspective on entropy—a measure of disorder—suggests that as the covalent bonds break, gallium’s atomic behavior becomes liberating.
This insight not only questions prior scientific dogmas but also opens doors for scientists to further explore how temperature manipulations can influence material properties. The potential ramifications of this knowledge extend towards innovations in nanotechnology, allowing researchers to experiment with gallium in developing new materials and processes.
A Glimpse into Gallium’s Applications
Gallium, often overlooked in the grand scheme of metals, plays a critical role in various high-tech applications, including semiconductor technology, telecommunications, and LED displays. As it serves as a substitute for mercury in thermometers, it provides a safer alternative while still delivering accurate readings.
Moreover, gallium’s potential has even piqued the interest of scientists searching for life signs on Mars. Its unique chemical properties may help unravel traces of past microbial existence, acting as a chemical “fingerprint” from eons past. This multifaceted applicability underscores gallium’s significance far beyond its physical peculiarities.
The Road Ahead: Bridging Science and Innovation
As the research led by Dr. Steph Lambie and colleagues continues to challenge existing paradigms, the implications extend far beyond theoretical discussions. A deeper comprehension of gallium’s behaviors and characteristics is poised to facilitate groundbreaking advancements not only in nanotechnology but also in various scientific sectors.
The monumental strides made in understanding gallium’s atomic structure signal a broader trend in science—one that embraces complexity while unraveling the intricacies of materials at the atomic level. As researchers continue to push boundaries, gallium stands as a testament to the idea that some of the most extraordinary capabilities lie in the most unassuming of substances. Embracing this journey of discovery could very well lead humanity towards a brighter future enriched with innovative materials and technologies.