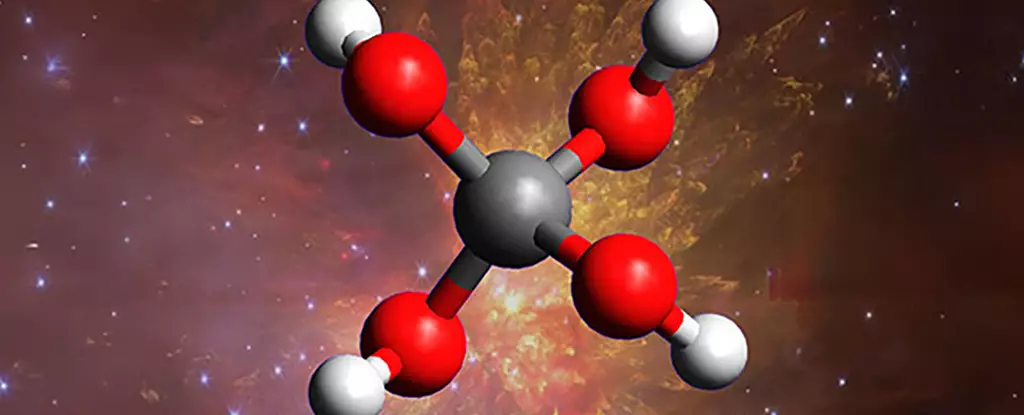
The universe continues to challenge our understanding of chemical processes, especially in the cold, dark regions between stars where conditions are seemingly inhospitable. Recent breakthroughs in laboratory simulations have revealed the existence of a molecule long predicted but never before observed: methanetetrol, a rare and complex “super alcohol” composed of a single carbon atom bonded to four hydroxyl groups (OH). This discovery not only confirms a century-old hypothesis but also expands the horizon of what molecules can form under extreme interstellar conditions, hinting at a universe far more dynamically chemical than previously imagined.
By mimicking the environment of deep space, scientists have achieved what was once considered impossible: the creation of a molecule that is inherently unstable on Earth. Through the precise freezing of carbon dioxide and water into ultra-cold ice within a vacuum chamber, and then exposing this artificial ice to high-energy radiation, researchers simulate the impact of cosmic rays and stellar activity. Their observations reveal that even in the depths of space, where molecules are thought to be simple, complex and unexpected chemical reactions can occur, producing molecular structures that defy our terrestrial expectations.
This discovery challenges the long-held notion that space chemistry is limited to basic molecules. Instead, it suggests a dynamic and nuanced chemical environment that can give rise to highly unstable, yet fascinating compounds. The fleeting nature of methanetetrol—breaking apart swiftly when exposed to light—has historically made it difficult to detect in nature. However, laboratory evidence clearly shows that the conditions in space could favor its transient formation, throwing open the question: are there many more such molecules lurking unseen in the cosmic backdrop?
Implications for Cosmic and Biological Evolution
The implications of finding methanetetrol extend beyond pure chemistry. It prompts us to rethink the molecular possibilities in interstellar clouds where star systems and planets are born. Compounds like methanetetrol may serve as intermediates or catalysts in more complex chemical pathways, potentially influencing the formation of prebiotic molecules that are essential for life as we know it. If such “impossible” molecules can form in space, then the chemical ingredients for life could be far more abundant and diverse than traditional models have suggested.
Scientists speculate that these complex molecules could act as building blocks, ultimately leading to the emergence of life-supporting systems elsewhere in the universe. The sudden realization that the interstellar medium hosts such exotic chemistry ignites excitement about discovering extraterrestrial life or life precursors in the far reaches of space. This chemical diversity bodes well for astrobiology, as it bolsters the idea that the universe is a fertile ground for molecules foundational to living organisms.
Furthermore, the discovery of methanetetrol presents an opportunity to refine our understanding of the physical conditions in space. Its instability under Earth’s light underscores the need for advanced detection methods capable of capturing fleeting molecular states in situ. As telescopic technology advances, such as the development of more sensitive spectrometers and imaging tools, astronomers stand at the threshold of detecting these elusive molecules directly in their cosmic habitats, offering a new window into the molecular universe.
Challenging Assumptions and Driving Future Exploration
The redefinition of space chemistry prompted by this breakthrough compels scientists to reevaluate current models of molecular formation in the cosmos. It is increasingly evident that the universe might host a far richer chemical tapestry than we have discovered so far. Currently, estimates suggest we have identified less than 1% of the molecules that exist in space, leaving vast uncharted territory ripe for exploration.
The laboratory recreation of methanetetrol hints at the possibility of other, even more exotic molecules that could exist fleetingly or in stable clusters under particular conditions. These molecules could influence physical processes such as star formation, dust grain chemistry, and even the evolution of planetary atmospheres. Recognizing the universe’s capacity to produce such complex molecules challenges existing dogmas and pushes scientists to develop more sophisticated models of space chemistry.
In particular, this research invigorates efforts in observational astronomy. The detection of short-lived molecules like methanetetrol requires exquisite sensitivity and rapid detection methods, pushing the boundaries of current technological capabilities. As new telescopes and instrumentation emerge, the potential to directly observe these molecules in star-forming regions or dense interstellar clouds becomes increasingly plausible, promising profound insights into the chemistry of the cosmos.
This progression compels a shift in scientific perspective: space does not merely host simple, inert molecules but an intricate and lively chemical ecosystem capable of producing astonishing, complex structures. As we deepen our understanding, the universe transforms from a barren void into an active chemical laboratory—one that continually surprises and defies our expectations.