Sphingolipids, a class of lipids crucial to various biological processes, first gained scientific attention at the end of the 19th century when German pathologist Ludwig Thudichum isolated them from brain tissues. He chose the name “sphingolipids” inspired by the enigmatic nature of the Sphinx, which seemed to pose numerous mysteries to researchers attempting to understand their complex roles within human health. Over the decades, numerous pathologies have been linked to irregular sphingolipid metabolism, including Fabry’s disease and Gaucher’s disease, but their ramifications extend beyond genetic disorders. In recent years, scientists have increasingly focused on sphingolipids in the context of infectious diseases, revealing their potential implications in conditions such as viral infections like COVID-19, Ebola, and Measles, as well as bacterial infections like Pseudomonas aeruginosa.
Critical to understanding sphingolipid dysfunction in these infectious contexts is the ability to visualize their metabolism effectively. One enzyme, sphingomyelinase, is instrumental as it catalyzes the breakdown of sphingomyelin—an essential component of sphingolipids—into bioactive molecules that participate in cellular signaling and inflammation. However, up until now, scientists lacked the means to visualize the activity of sphingomyelinase in living cells or during infection processes, effectively hampering advances in both basic and applied research.
Recent Advancements in Visualization Techniques
In a landmark study published in *Nature Communications*, a collaborative team of researchers from Würzburg and Berlin have successfully synthesized a novel sphingomyelin derivative designed specifically to visualize sphingomyelin metabolism and sphingomyelinase activity. This innovation opens new avenues for understanding the metabolic processes underlying various infectious diseases. The research falls under the initiative of the Research Training Group 2581, where a multidisciplinary team of chemists, biologists, and physicists put their expertise together to overcome longstanding challenges in infection research.
The trifunctional sphingomyelins developed by the research team represent a significant leap forward; they are crafted to closely mimic natural sphingomyelin yet have been augmented with additional capabilities for tagging and visualization. This molecular adaptation allows researchers to monitor the localization and degradation of sphingomyelin in real-time, providing unprecedented insight into the role of sphingomyelin metabolism in infections.
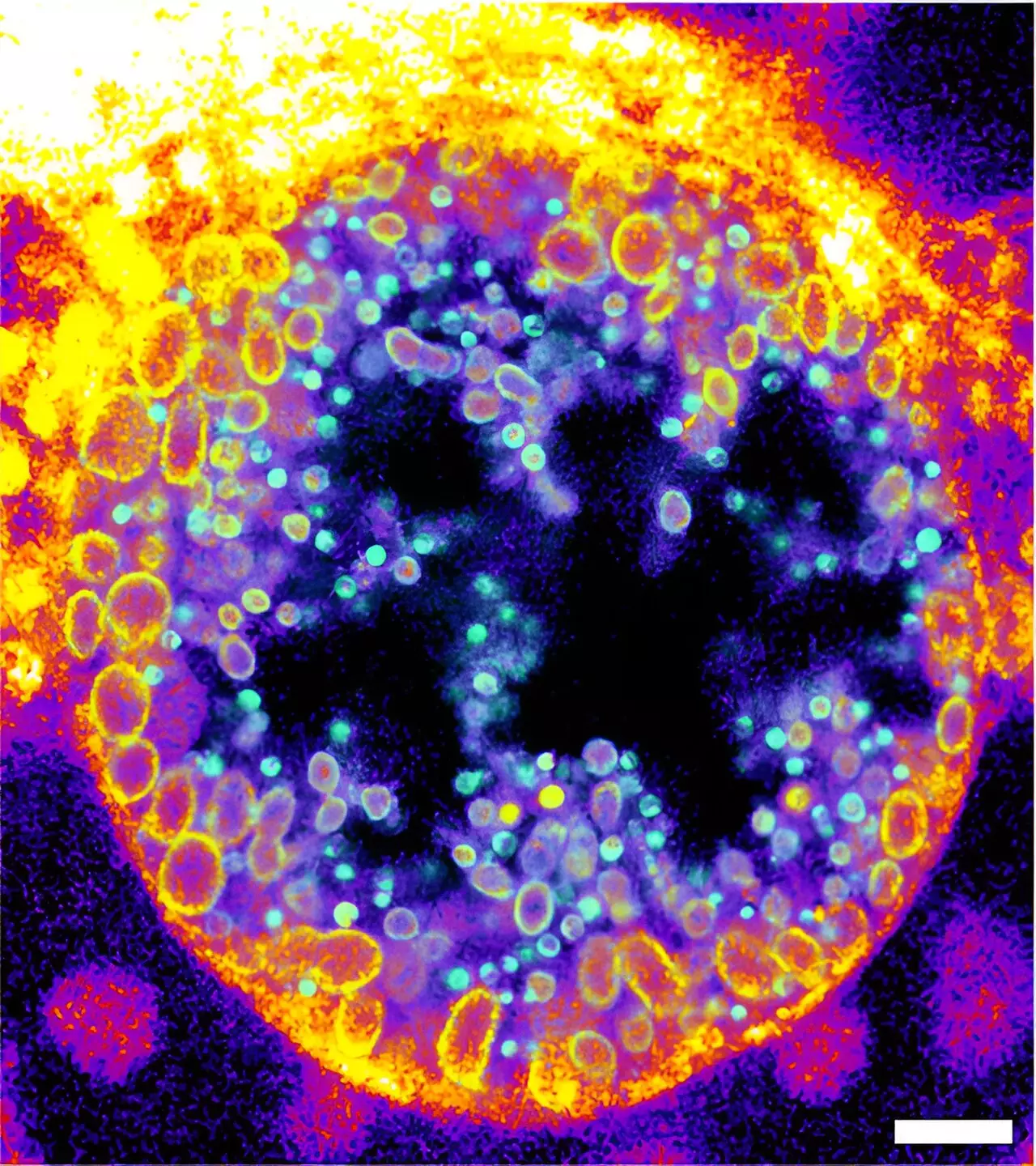
The researchers conducted detailed experiments to validate the functionality of their newly engineered molecules. They employed various methods—including expansion microscopy and click-chemistry—to demonstrate the metabolic products associated with bacterial sphingomyelinase activity on human cell surfaces. Intriguingly, their analysis uncovered that during the infection cycle of Chlamydia bacteria—recognized for their pathogenicity in the human genital tract and their potential link to cancer—there was a clear correlation between the maturation of infectious particles and the metabolism of sphingomyelin.
During their studies, the team noted that chlamydial inclusions within infected cells primarily accumulated cleaved forms of the trifunctional sphingomyelins, demonstrating a significant increase in metabolized sphingomyelin over the course of the infection. These findings suggest that sphingomyelin plays a dynamic role during infectious processes, with its degradation being closely tied to the life cycle of the pathogen.
Implications for Future Research
The implications of this research are profound. With the ability to visualize sphingolipid metabolism and sphingomyelinase activity, scientists can develop targeted therapeutic strategies that manipulate sphingolipid pathways during infections. Not only does this enhance our understanding of existing infectious diseases, but it may also lead to new approaches for the treatment and possibly prevention of conditions accentuated by sphingolipid abnormalities.
As reiterated by Professor Jürgen Seibel of the Institute of Organic Chemistry at Julius-Maximilians-Universität, this innovative chemical tool has a broad potential for application in various laboratories, capable of serving as a foundation for future research endeavors aimed at tackling infections at their metabolic roots.
The development of this new molecule for visualizing sphingomyelin metabolism marks a significant step forward in infection research and sheds light on the critical role of sphingolipids in health and disease. As the scientific community continues to unravel the complexities of lipid metabolism, groundbreaking discoveries such as these can pave the way for transformative therapeutic strategies in the fight against infectious diseases.