The oxygen evolution reaction (OER) is a fundamental electrochemical process crucial for renewable energy applications, particularly in water splitting and metal-air batteries. Catalysts that facilitate OER play an essential role in improving the efficiency of these technologies by lowering the energy barriers for the reactions involved. Recent research focused on developing cost-effective and high-performance catalysts has shown promising results, indicating a significant stride toward enhancing the viability of green energy solutions.
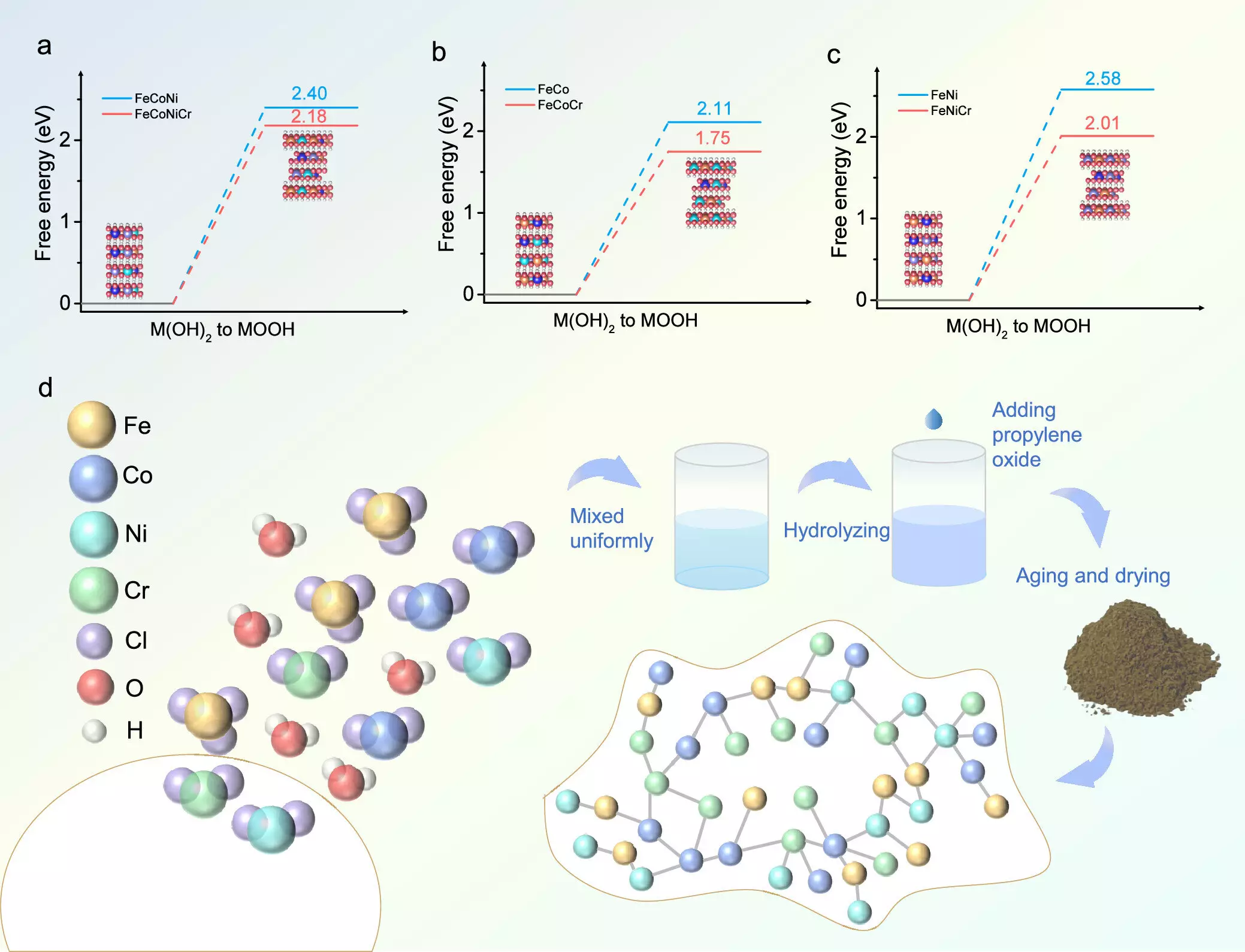
A groundbreaking study recently highlighted the benefits of incorporating chromium (Cr) into transition metal hydroxides to enhance their catalytic performance. Researchers utilized a dual approach comprising density functional theory (DFT) calculations and experimental methods to synthesize a versatile FeCoNiCr hydroxide catalyst. This innovative catalyst demonstrated improved reaction kinetics, essential for effectively driving the OER.
Hao Li, a prominent researcher at Tohoku University’s Advanced Institute for Materials Research, emphasized the impact of chromium doping on the structural dynamics of metal hydroxides. The introduction of Cr was crucial for expediting the phase transition to an active oxyhydroxide state, thereby amplifying the OER efficiency.
The experimental work conducted by the research team unveiled impressive performance metrics for the synthesized catalyst. In alkaline environments, the FeCoNiCr hydroxide exhibited an exceptional low overpotential of 224 mV, surpassing similar catalytic systems by 52 mV. Furthermore, the catalyst’s stability was put to the test over 150 hours of continuous operation, showcasing its durability and performance consistency.
Another significant outcome from this research was the effective utilization of the catalyst in a zinc-air battery, which managed to operate stably for 160 hours while maintaining a remarkably low voltage difference during charge-discharge cycles. These metrics strongly indicate that the new catalyst can not only catalyze the OER efficiently but also function reliably in practical applications.
Theoretical analyses supported the experimental observations, indicating that chromium effectively tunes the electronic environment of the active sites, thereby optimizing the adsorption energies of the OER intermediates. Bader charge analysis revealed that both nickel and cobalt predominantly retained a +3 oxidation state during the OER, contributing to sustained catalytic activity.
Moving forward, the research team aims to explore various elements that could further enhance catalyst performance. Di Zhang, a co-author of the study, highlighted the potential of their methodology for efficiently screening and designing advanced catalysts. This research serves as a foundation for developing even more effective catalysts that could expedite the adoption of clean energy technologies, particularly in hydrogen production.
As the global quest for renewable energy intensifies, the breakthroughs in catalyst development for the OER stand as a beacon of hope. Innovations like chromium doping in transition metal hydroxides not only enhance performance but also lay the groundwork for efficient and cost-effective energy solutions. The ongoing efforts in this field are crucial to advancing the implementation of clean energy technologies and fulfilling rising energy demands sustainably.