The recent awarding of the Nobel Prize in Chemistry has marked a significant milestone in the field of molecular biology, spotlighting groundbreaking work on proteins, the essential components of life. The distinction was granted to three trailblazing scientists: Demis Hassabis and John Jumper from Google’s DeepMind lab, along with the biochemist David Baker. Their combined efforts have propelled our understanding of proteins into uncharted territories, paving the way for innovations that could transform medical treatments and environmental solutions alike.
While the application of artificial intelligence (AI) has long been confined to computational tasks, Hassabis and Jumper have ingeniously harnessed it to tackle one of biology’s most complex puzzles: protein folding. Their AI model, AlphaFold, has revolutionized how scientists predict the three-dimensional structures of proteins by utilizing an extensive database of known amino acid sequences and their respective configurations. Meanwhile, Baker’s innovative approach of engineering entirely new proteins exemplifies a different facet of science that could redefine our understanding of molecular interactions.
To comprehend the significance of the Nobel-winning work, it’s paramount to understand what proteins are and why they are indispensable to all living organisms. Proteins are large, complex molecules composed of chains of amino acids that perform a myriad of crucial functions within the body, from catalyzing biochemical reactions as enzymes to providing structural support to cells. Essentially, proteins are the machinery that enables cells to perform their functions as dictated by the genetic blueprint encoded in DNA.
As Mary Carroll from the American Chemical Society analogizes, envisioning the linear sequence of amino acids as an uncoiled telephone cord underscores the intricate architecture of proteins. The sequence of amino acids ultimately dictates how these molecules will fold into specific three-dimensional shapes, which is vital for their functionality. This understanding posits that if researchers could solve the coding riddle of protein sequences, they could engineer proteins tailored for specific tasks. This has been a longstanding ambition of chemists, but one fraught with challenges and missteps.
Despite past endeavors and the introduction of methodologies like the Protein Olympics — a competition aimed at assessing protein structure predictions — success in this field has proven elusive. For over fifty years, scientists grappled with the intricate details of translating linear amino acid sequences into functional 3D structures. Many attempts fell short of accurately predicting these configurations, leading to a stagnation in progress.
Enter Hassabis and Jumper. Their foresight in employing AI techniques to decode protein folding has been heralded as a quantum leap in understanding molecular biology. The advantage of AlphaFold lies in its ability to compare unknown protein sequences against a vast repository of existing data, constructing probable three-dimensional shapes through machine learning algorithms. AlphaFold2’s triumph in the 2020 Protein Olympics signaled a pivotal moment, as its accuracy overshadowed previous methods and positioned AI as a reliable, transformative tool in protein science.
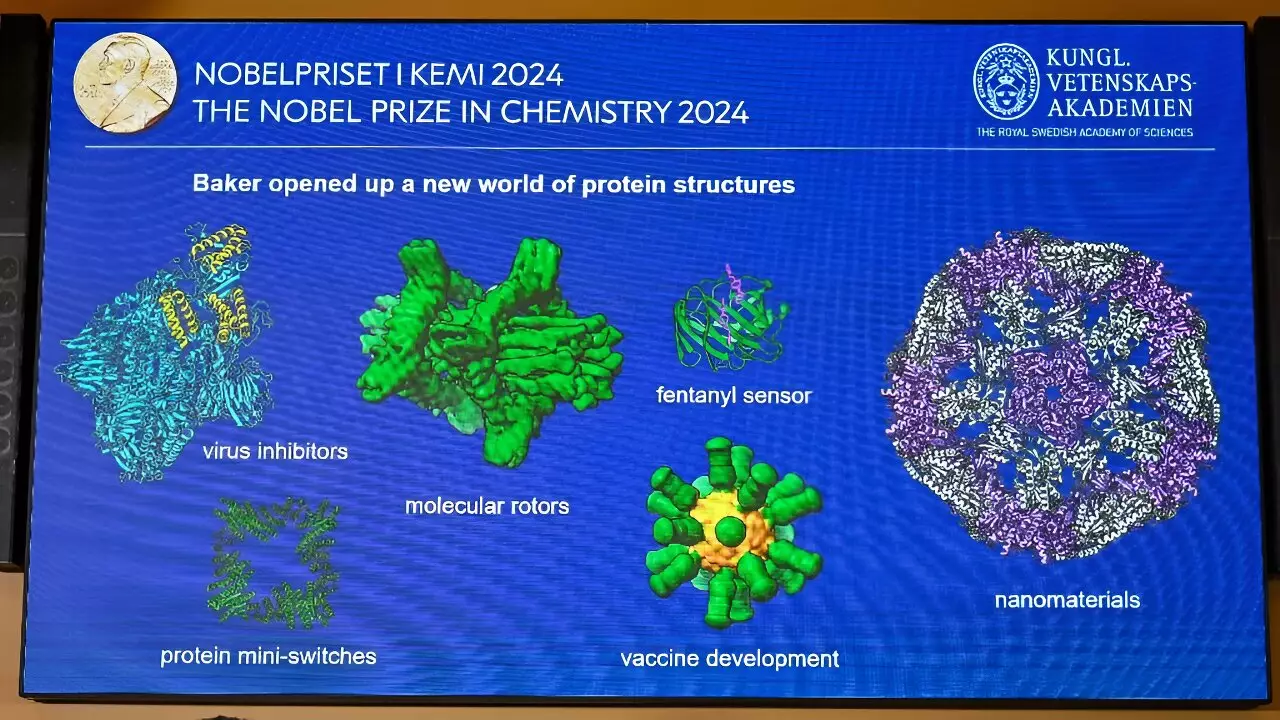
In parallel to the predictive advancements offered by AlphaFold are Baker’s endeavors in protein design. By developing a computational method named Rosetta, Baker can not only predict structures but also create novel proteins that do not exist in nature. This approach entails an exploration of thousands of protein structures to find fragments that resemble the desired outcome. Such an innovative strategy brings forth the possibility of designing proteins for specific applications, ranging from novel therapeutic agents to advanced materials.
Baker’s commitment to protein engineering is particularly exciting given the potential ramifications for future healthcare solutions. During the Nobel ceremony, he highlighted a protein designed for the pandemic protection against the coronavirus, symbolizing a step towards future vaccines and therapies that could preemptively counter viral outbreaks.
The implications of understanding proteins more comprehensively extend beyond theoretical knowledge; they carry the promise of practical applications that could revolutionize multiple sectors. From developing targeted treatments for diseases to engineering microbes capable of breaking down pollutants, the possibilities seem boundless. As noted on the Nobel Prize website, mastering protein design could lead to the creation of new nanomaterials, drugs, vaccines, and environmentally-friendly chemicals.
As we stand on the brink of this new frontier in protein science, the insights gained from the award-winning research are likely to catalyze an era of unprecedented advancements in medicine and biotechnology. The excitement voiced by protein researcher Davide Calebiro underscores a future filled with hope and potential, where the secrets of life can be further unraveled, and the mysteries of health and disease addressed with enhanced precision.
With this prestigious accolade, the scientific community can expect a surge of inspired research aimed at exploring the complex interplay of amino acids, structures, and life itself—an adventure just beginning.