The quest for sustainable energy sources has taken on renewed urgency in recent years, with hydrogen production emerging as a crucial player in the transition towards greener technologies. Among various methods to produce hydrogen, the electrolytic splitting of water stands out, particularly through photoelectrochemical cells (PEC). These systems utilize photoelectrodes to harness sunlight, converting it into the electrical energy necessary for electrolysis. Recent advancements indicate that operational pressure can significantly enhance the performance of these cells, making them more efficient in their role to contribute to sustainable energy solutions.
At the core of PEC cells lies the ability to mimic natural processes found in plants. Traditionally, nature uses a complex known as Photosystem II to convert light energy into chemical energy, splitting water molecules into hydrogen and oxygen. PEC technology aims to replicate this functionality using artificial and inorganic materials that can effectively generate the required voltage for water electrolysis. Research has demonstrated that some of these devices can achieve energy conversion efficiencies as high as 19%. However, at these elevated levels, inefficiencies arise, primarily due to the formation of gas bubbles during the electrolysis process.
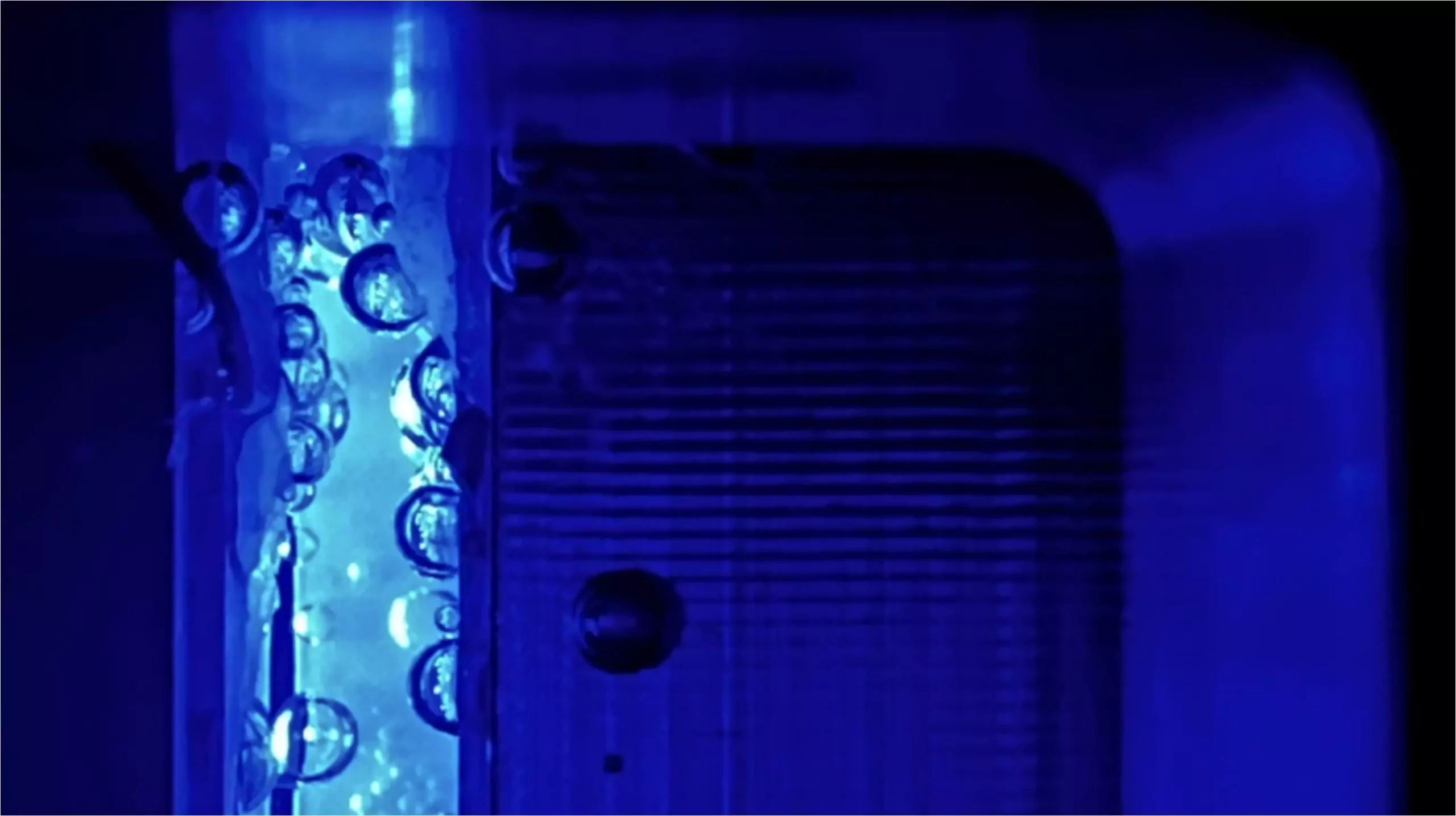
Gas bubbles present a significant challenge in PEC systems. They not only scatter incoming light, reducing the effective illumination of the electrodes but can also obstruct the contact between the electrolyte and the electrodes, causing a drop in electrochemical performance. To mitigate these issues, the operational environment of PEC cells can be modified—in particular, exploring the impact of elevated pressure on bubble dynamics and overall system efficiency.
The recent study conducted by a research team at the Helmholtz Centre Berlin (HZB) takes a deep dive into the effects of higher pressure within PEC cells. By utilizing gas to pressurize these systems between 1 and 10 bar, the researchers sought to understand how this alteration would impact energy losses due to bubble formation. Their comprehensive approach involved a multiphysics model to simulate the process, allowing them to analyze various parameters affecting efficiency.
The findings reveal promising potential. By increasing the pressure to 8 bar, the researchers identified that overall energy loss could be reduced by half, resulting in a possible 5–10% increase in efficiency. This significant improvement stems from the fact that higher pressures effectively minimize optical scattering losses, enhancing the functional performance of the photoelectrodes. Additionally, the study observed a noteworthy decrease in the unwanted transfer of oxygen to the counter electrode, further optimizing the system’s efficiency.
Interestingly, the research concluded that pushing the pressure beyond 8 bar did not yield further advantages, suggesting there is an optimal operating range between 6 and 8 bar for PEC electrolyzers. Thus, while pressure has shown to be a crucial variable, it must be finely tuned to balance energy losses and maximize conversion efficiency. The ability to manipulate key parameters, as demonstrated through the multiphysics model, opens up avenues for similar advancements in other electrochemical and photocatalytic systems.
Beyond the technical advances presented by the HZB team, the implications of this research extend into the operational efficiency of renewable energy technologies. As hydrogen production plays a pivotal role in future energy infrastructures—such as fuel cells and energy storage systems—enhancing the performance of PEC cells represents a significant step forward in developing sustainable practices.
As we stand at the intersection of energy innovation and environmental responsibility, ongoing research in areas like PEC efficiency remains paramount. The findings from HZB illustrate not only how far we have come in harnessing renewable energy sources but also highlight the potential for much more in the future, paving the way for a more sustainable and energy-efficient world.