Ammonia (NH3) stands out as a fundamental compound in both agricultural and industrial sectors. Boasting a staggering global market size of around 175 million metric tons and a valuation of approximately $67 billion, ammonia plays an irreplaceable role in the production of fertilizers that feed a growing global population. Beyond agriculture, ammonia serves as a building block for various chemicals and materials, underscoring its significance in the industrial landscape. The rise of the hydrogen economy further amplifies ammonia’s relevance, positioning it as a high-energy-density carrier that could facilitate the seamless transportation and storage of hydrogen.
Despite its importance, the conventional methods of ammonia production, primarily through the Haber-Bosch process, present substantial drawbacks. This traditional technique demands high energy inputs and results in considerable carbon dioxide (CO2) emissions, drawing scrutiny in an era increasingly focused on sustainability. As industries grapple with climate commitments, the pressure to innovate cleaner ammonia production methods has never been more intense. This context sets the stage for exciting advancements in electrochemical synthesis of ammonia from nitrate (NO3–), a breakthrough led by researchers at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR).
Recent findings from Hao Li and his research group reveal the potential to transform ammonia production through the electrochemical conversion of nitrate to ammonia. Unlike nitrogen fixing, which involves the challenging cleavage of the N=N triple bond in molecular nitrogen, nitrate reduction offers a smoother, more favorable chemical pathway. Nitrate features lower dissociation energy and improved water solubility, positioning it as an ideal nitrogen source. This innovation not only enhances ammonia synthesis efficiency but also mitigates environmental concerns related to nitrate surplus in aquatic ecosystems.
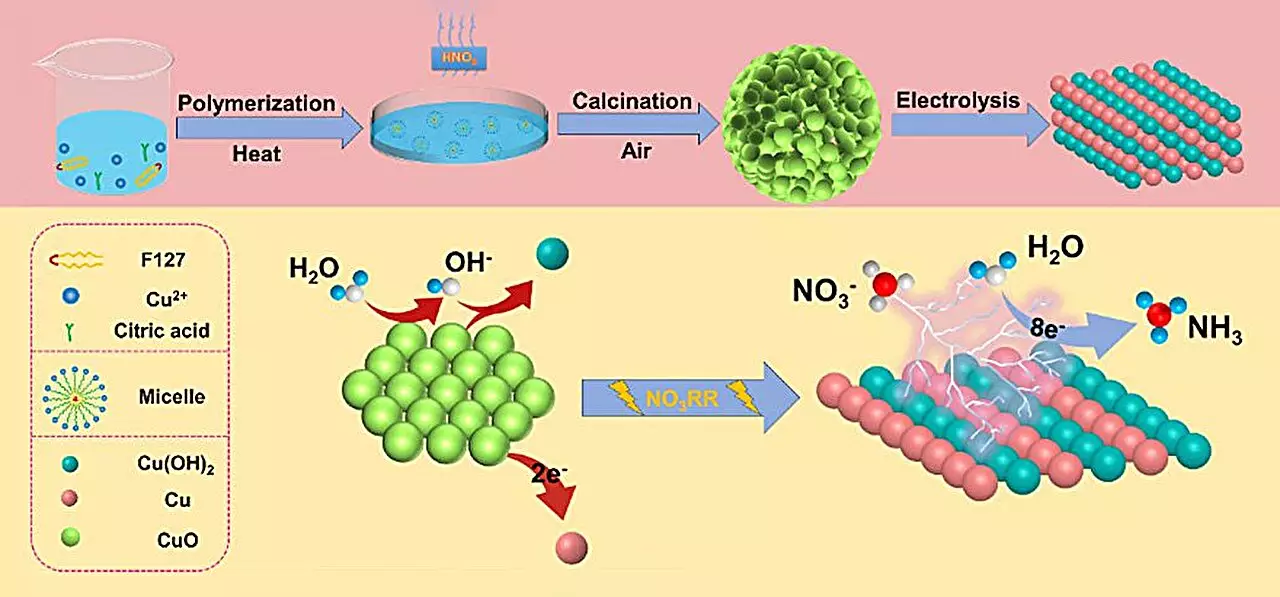
The researchers developed a spherical copper (II) oxide (CuO) catalyst designed for superior ammonia yield. This catalyst, characterized by tightly stacked small particles containing oxygen-rich vacancies, achieved notable results: an ammonia production rate of 15.53 mg h-1 mgcat-1 and an impressive Faraday efficiency of 90.69% at a voltage of -0.80 V against a reversible hydrogen electrode. Such results underscore the catalyst’s remarkable efficiency and the importance of catalyst design in the quest for sustainable chemical synthesis.
According to co-author Qiuling Jiang, a significant reason behind the catalyst’s extraordinary performance lies in its transformative phase changes during the electrochemical process. As CuO undergoes structural transformation to form a Cu/Cu(OH)2 composition, the number of active sites increases, facilitating superior electron transfer. This interplay of structural changes appears to be pivotal in optimizing the nitrate reduction reaction.
A further layer of innovation emerges from density functional theory (DFT) calculations, which the research team employed to elucidate the underlying catalytic mechanisms. Their analysis illustrated how the generation of Cu(OH)2 reduces the activation energy required for nitrate adsorption, thus favorably positioning the process toward efficient ammonia production. Additionally, this Cu(OH)2 phase actively suppresses unwanted hydrogen evolution reactions, while the presence of Cu (111) crystal surfaces enhances hydrogenation. This understanding pivots the design of copper-based catalysts for more effective electrochemical ammonia production.
As this pioneering research unfolds, the team expresses aspirations to delve deeper into the factors influencing the phase transitions of their catalysts during the nitrate reduction process. By optimizing catalyst design for higher stability, activity, and selectivity, the goal becomes clearer: to establish a more efficient and environmentally conscious framework for ammonia synthesis.
In the broader context of sustainable development, such advancements not only promise to revolutionize ammonia production but also align with global efforts toward reducing carbon footprints and addressing climate change. The exploration of innovative electrochemical routes stands as a beacon of hope, spotlighting the potential for a cleaner, more sustainable future in chemical manufacturing.