As the world grapples with the dire consequences of climate change, researchers are turning their attention to innovative strategies to mitigate carbon emissions, particularly carbon dioxide (CO2). A promising avenue that has emerged in this pursuit is electrochemical reduction, a process wherein electrical energy is harnessed to convert captured CO2 into useful products such as methanol and ethanol. This article will delve into the recent advancements made by a team of researchers from the U.S. Department of Energy’s (DOE) Brookhaven National Laboratory, Yale University, and the University of North Carolina at Chapel Hill, highlighting their significant progress in enhancing catalyst efficiency.
The heart of the electrochemical reduction process lies in the efficiency of catalysts—substances that speed up chemical reactions without being consumed in the process. Traditional catalysts often require substantial energy inputs, raising concerns about their practicality for large-scale applications. Researchers have long sought catalysts that can not only sustain the reduction of CO2 but do so quickly and with minimal external energy. The Brookhaven-led team has made remarkable strides in this regard, with their recent research resulting in a catalyst that accelerates reaction speed by an astonishing factor of 800.
The importance of this finding cannot be overstated. By identifying a catalyst that operates with reduced energy requirements, the researchers have opened the door to more economically viable carbon capture techniques. “A major barrier we’ve encountered in deploying catalysts at scale is their energy consumption,” noted Gerald Manbeck of Brookhaven, who contributed to the study. The research offers not only a solution to this issue but also a foundation for designing advanced catalysts in the future.
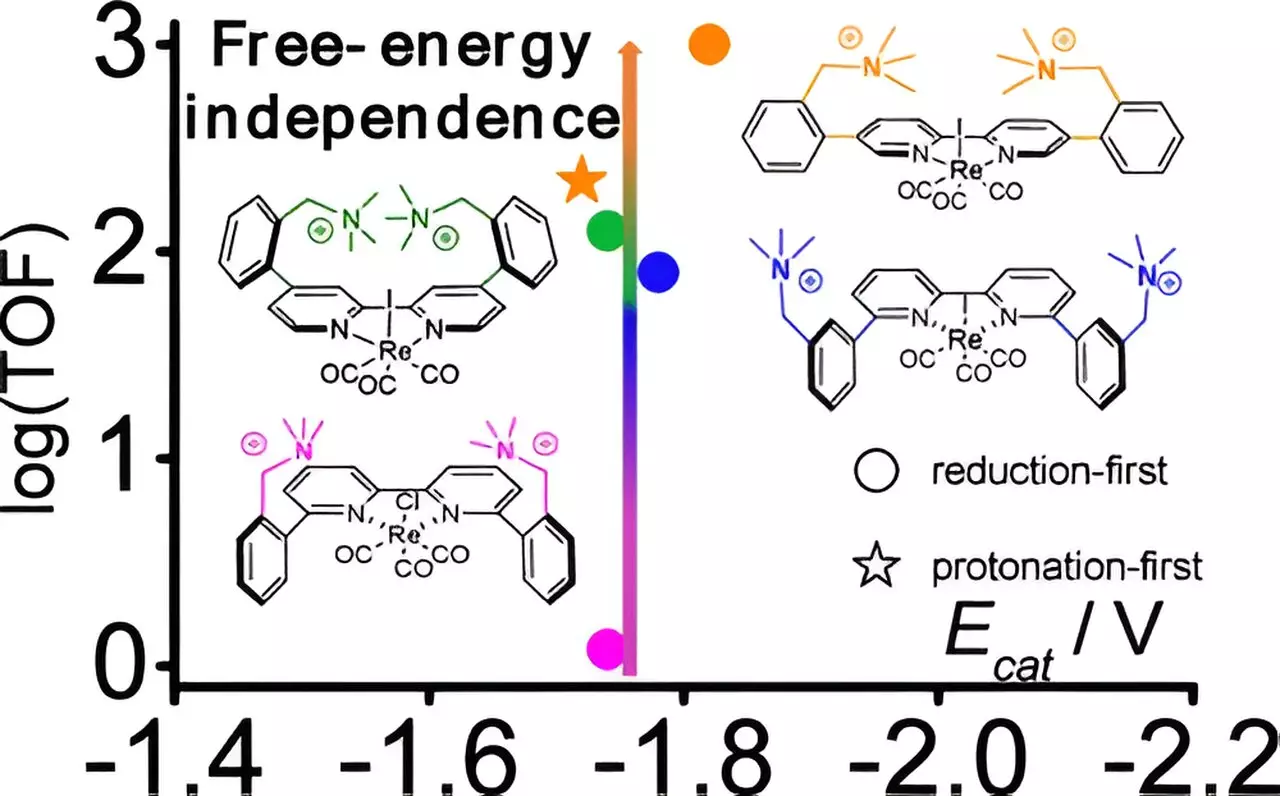
The team’s research began with an exploration of existing catalysts, focusing particularly on those based on rhenium, a rare and valuable metal. The catalyst they investigated consists of rhenium atoms supported by various organic fragments composed of carbon, nitrogen, oxygen, and hydrogen. The breakthrough came when the researchers experimented with different cation configurations—positively charged molecules that can stabilize the catalytic process. By adjusting the spatial arrangement of these cations in relation to the rhenium center, the team discovered a specific configuration that significantly enhanced the catalytic efficacy.
Through the application of computational chemistry principles, the researchers elucidated the roles these cations play in stabilizing intermediate reaction states. Their systematic approach unveiled a previously unrecognized low-energy pathway for rhenium-based catalysts, presenting a distinct opportunity for reducing the energy barrier typically encountered in CO2 reduction.
The researchers employed a variety of advanced analytical techniques to substantiate their findings. Cyclic voltammetry allowed them to quantitatively assess the energy dynamics and reaction rates, while infrared spectroelectrochemistry provided insights into the structural transformations of the reactants during the electrochemical process. The sensitivity of the apparatus they developed has been instrumental in observing chemical alterations occurring at the interface between the solution and electrodic surface, thereby enriching their understanding of the catalytic mechanism.
This carefully orchestrated series of experiments not only validated their theoretical predictions but also illuminated the significance of even minute geometric modifications in catalyst design. “Our findings reveal the impact of structural changes on catalytic performance, emphasizing how subtle adjustments can lead to substantial improvements,” stated Laura Rotundo, the lead author of the research paper.
Looking ahead, the research team is keen on refining their catalytic system by introducing semiconductor light absorbers, such as silicon, which have the potential to harness sunlight and convert it into electrical energy. The idea is to explore whether these light absorbers could partially drive the catalytic process, decreasing reliance on traditional electrical inputs. This future direction aligns seamlessly with the broader mission of the Carbon-Hydrogen Sustainable Energy (CHASE) initiative, which seeks to innovate photoelectrodes capable of efficiently converting CO2 and water into liquid fuels by utilizing solar energy.
The interplay between innovative catalytic design and renewable energy sources emphasizes a transformative approach to CO2 reduction. The achievements of the Brookhaven-led research team can catalyze further exploration and development in this field, ultimately contributing to cleaner and more sustainable energy solutions. Such initiatives not only advance scientific understanding but also represent a critical step toward combating climate change and fostering a greener future.