Photocatalysis is a transformative field inspired by natural phenomena, particularly photosynthesis, where light energy is harnessed to facilitate chemical reactions. Traditionally, certain reactions require either high temperatures or aggressive conditions; however, photocatalysts can lower those barriers, producing a more accessible pathway for various chemical transformations. For these methodologies to achieve practical relevance, especially in industrial applications, it is crucial that the quantum efficiency—the measure of how effectively light is converted to chemical energy—is maximized.
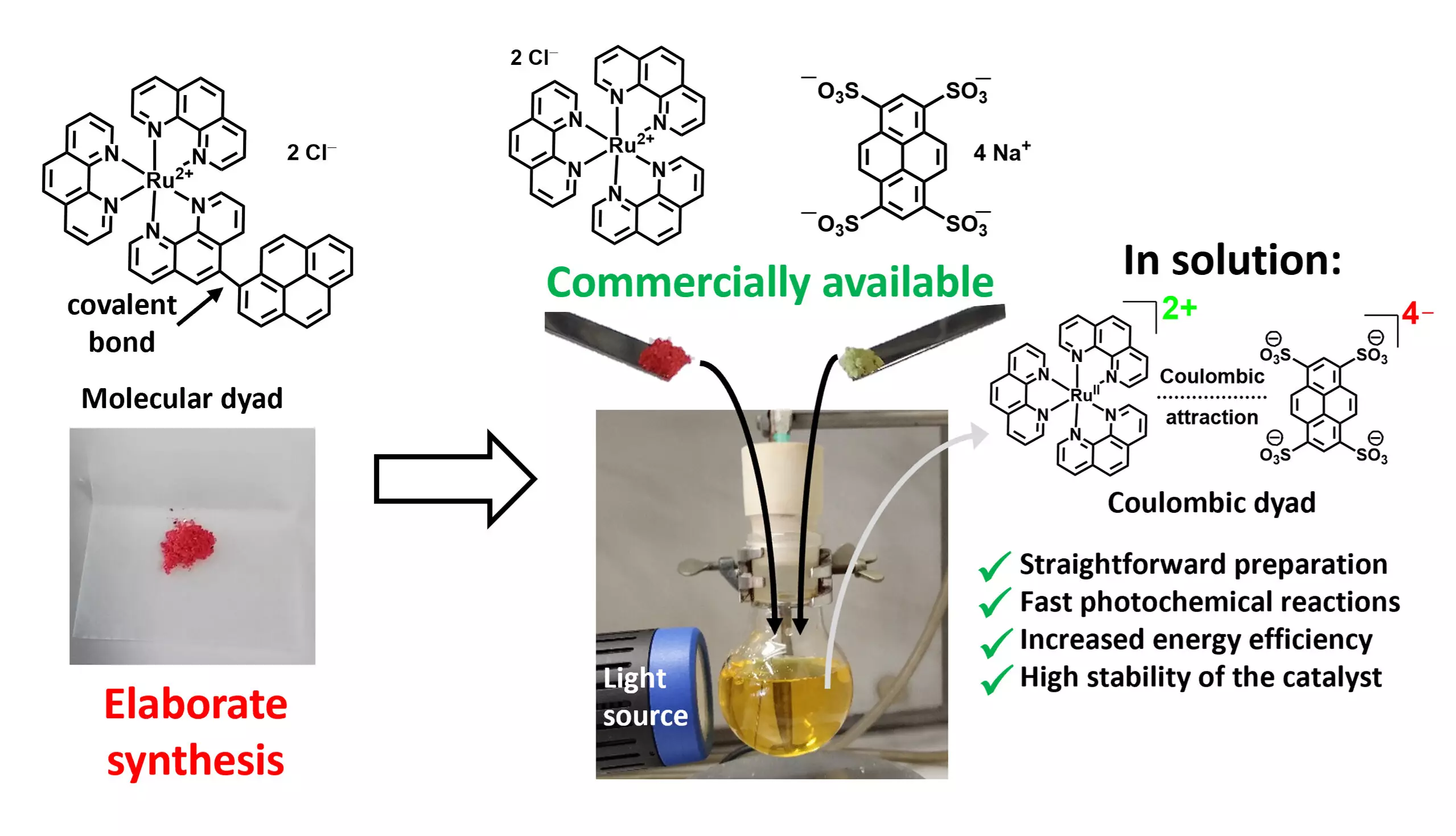
The research led by Professor Christoph Kerzig at Johannes Gutenberg University Mainz (JGU) showcases a pivotal evolution in photocatalyst development. Utilizing what they refer to as Coulombic dyads, these novel catalysts combine two different photoactive units through electrostatic interactions, thus eliminating the need for the extensive multi-step synthesis often required for traditional dyads. This advancement represents a significant step towards making photocatalysis economically viable for large-scale applications.
From Complexity to Simplicity
Developing efficient photocatalysts has often involved intricate and costly synthesis processes, particularly when high-performance catalysts are constructed from precious metals such as iridium or ruthenium. Researchers are constantly attempting to develop Earth-abundant alternatives. However, the complexities involved—due to the need for precise ligands and resource-intensive syntheses—have impeded progress in creating viable, low-cost photocatalysts.
The groundbreaking approach described by Kerzig and his team enables the formation of efficient photocatalysts using commercially available metal salts, attracting attention for reducing overall production costs. Lead researcher Matthias Schmitz emphasizes that by mixing specific salts, researchers can leverage the inherent Coulomb interactions to form ion pairs of photoactive materials, allowing for synergistic interaction. This method simplifies the process remarkably and positions it as a promising strategy for enhancing photocatalytic efficiency.
The discovery’s implications span various scopes of application and relevance. The initial experiments with these dyads involve notable chemical processes such as forming carbon-carbon bonds and performing photooxygenation on bio-derived materials. Not only do these reactions converge on creating valuable chemical products, but they also highlight the improved efficiencies when using Coulombic dyads compared to their more expensive counterparts.
The experimental results indicate a material enhancement in catalytic activity and photon conversion capabilities—key advantages that can drive forward the use of alternative energy sources like sunlight and LED-generated light in chemical reactions. The shift reflectively aligns with global sustainability initiatives by potentially providing greener and lower-cost alternatives to traditional chemical processes.
An integral part of this research is understanding the dynamic roles solvents play in influencing the efficiency of the reactions. The researchers propose the concept of a “toolbox” of different formulations; by varying the combinations of photoactive anions and cations, they can design distinct Coulombic dyads tailored for specific reactions or performance criteria. This customizable approach not only makes the photocatalysts versatile but also opens avenues for optimizing light-driven reactions on an industrial scale.
Their findings could ultimately reshape how many industries approach photocatalytic processes. With a method that relies on inexpensive components, the goal is to enhance the performance of existing metal-based catalysts and potentially decrease the material costs needed to catalyze chemical reactions.
Looking Ahead: The Future of Photocatalysis
The implications of this research are profound. As the team continues to refine their findings, they hope to pioneer a new foundation for industrial photoreactions. With ongoing efforts aimed at broadening the range of applications and optimizing catalyst properties, this innovative strategy paints a hopeful picture for the future of sustainable chemistry. As photocatalysis steps towards a more accessible and cost-effective frontier, the opportunity to harness light for a multitude of chemical transformations could fundamentally alter the landscape of material production and environmental impact. Through these advancements, we approach a more sustainable chemical industry that balances efficacy and affordability.