Cancer remains one of the most formidable challenges in modern medicine, primarily due to its inherent complexity and the ability of cancer cells to proliferate uncontrollably. Central to halting this unchecked growth is the strategic understanding of the proteins that allow cancer cells to survive and thrive. Identifying specific proteins and their interactions can give researchers a clearer target for the next generation of cancer therapies. An advanced layer of this quest involves protein profiling, a methodology pivotal for highlighting potential protein targets that new drugs should aim to disrupt.
Despite the promise of protein profiling, traditional methods have yielded limited insights. Conventional techniques often lack the nuanced detail required to expose every possible protein target, often leading to significant discoveries being overlooked. Recent advancements, however, are pushing boundaries. Researchers from Scripps Research have introduced a breakthrough by integrating multiple methods of protein analysis, resulting in the mapping of over 300 cancer proteins alongside their binding sites for small molecules—components crucial for halting cancer cell growth.
The Scripps Research team’s innovative work is rooted in the activity-based protein profiling (ABPP) technique—an approach invented by co-senior author Benjamin Cravatt. This technique enables scientists to capture and analyze protein activity on a global scale. The dual-method strategy employed by the researchers showcases the strength of combining various analytical methods to capture a more comprehensive landscape of protein behavior in response to targeted compounds.
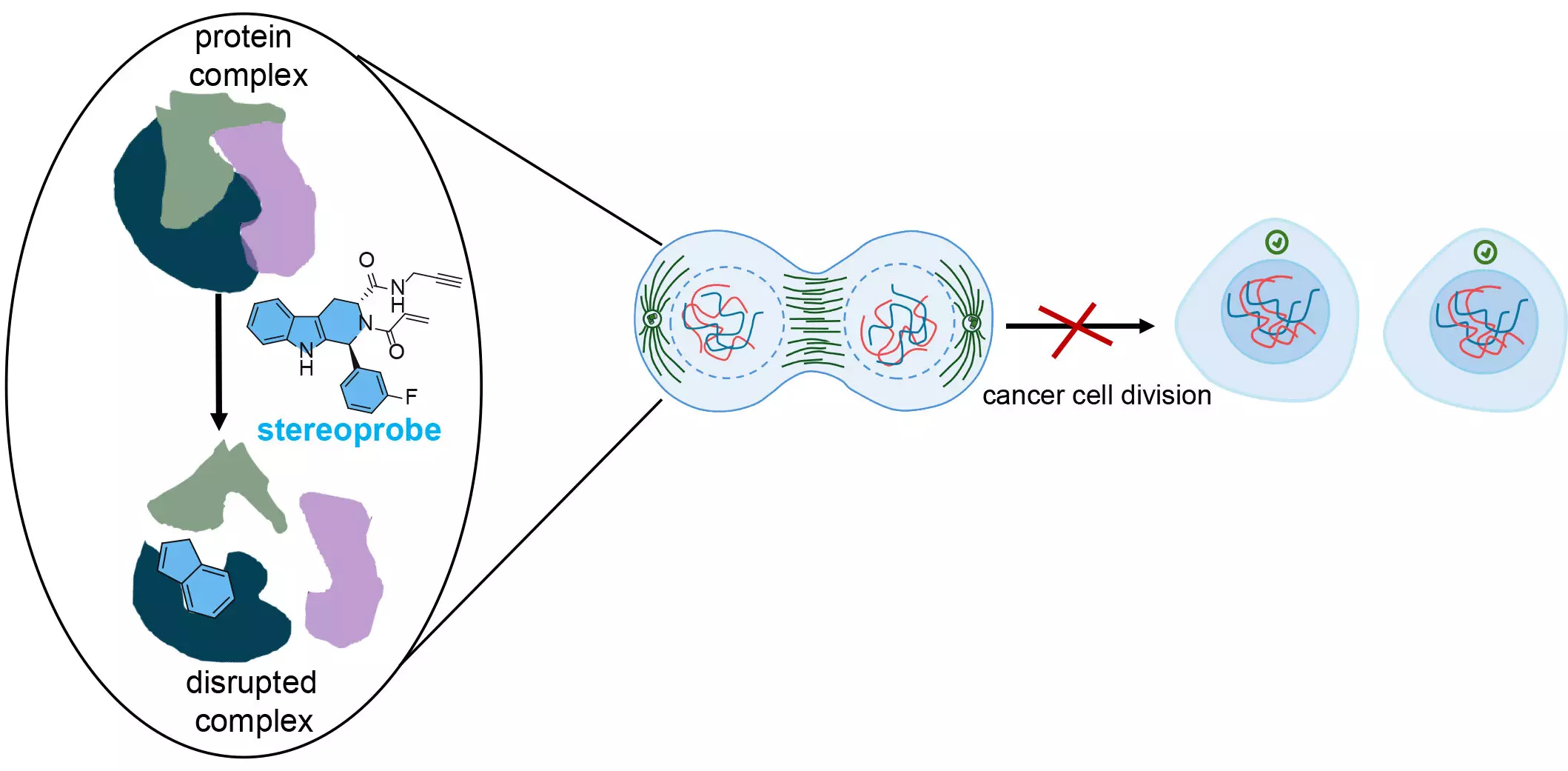
By deploying two distinct protein analysis techniques, the research team was able to create a detailed map of protein interactions with a library of stereoprobes—chemical compounds designed for selective and permanent protein binding. These stereoprobes were meticulously engineered to include chemical attributes that are often underutilized in standard drug discovery applications. The deliberate design choices made by the researchers enhance the potential for discovering new insights that can lead to advancements in biomedical science and improved health outcomes.
A critical focal point of this research is the amino acid cysteine. Known for its nucleophilic nature, cysteine plays a fundamental role in the structural integrity of numerous proteins, including those associated with cancer. The team’s stereoprobes are engineered to interact specifically with cysteine, presenting a unique opportunity to disrupt the functional architecture of cancer proteins. When a chemical modifies these cysteine residues, the resulting disruption can impede protein interactions essential for cancer cell proliferation, effectively hindering growth.
The dual approach of identifying both the proteins and the exact sites of interaction allowed the researchers to analyze these chemical bindings deeply. The technique known as cysteine-directed ABPP not only offers insight into which proteins are affected but also reveals the precise mechanism of action, akin to identifying the fitting piece in a complex puzzle. This granularity of data is essential in developing therapies that can target cancer cells with high specificity.
The implications of these findings are profound. By accurately identifying protein regions critical to cancer cell survival, there lies enormous potential for creating targeted treatments that specifically thwart the processes enabling cancer proliferation. The core insight from this research is the superiority of a combined analytical approach, which has highlighted the significant loss of critical protein targets that may occur when relying solely on a single method of analysis.
The insights gained from this study point towards a future in which cancer therapies could be refined to disrupt specific cell cycle stages—effectively putting cancer cells in a state where they are recognized by the body’s immune system as defective and subsequently prompted to die.
As the research team looks forward, they express interest in extending their work beyond oncology, emphasizing the need for stereoprobes that can be customized for diverse health challenges, including inflammatory diseases. Numerous proteins are implicated in various pathologies, and the creation of new stereoprobe libraries holds promise for unraveling the complexities of these conditions.
The work being published in Nature Chemistry represents not just an advancement in cancer research but serves as a testament to the power of interdisciplinary approaches in unraveling the intricacies of life at a molecular level. Through continued innovation and exploration, researchers like those at Scripps Research are positioning themselves at the forefront of transformative cancer treatment paradigms, potentially redefining how we approach and manage this prolific disease in the future.