Water pollution remains one of the most pressing environmental issues, particularly when it comes to the contamination of aquatic ecosystems and the safety of drinking water. Heavy metals, including cadmium and lead, are notorious for their toxicity, posing serious health risks to both humans and marine life. The challenge lies in effectively removing these contaminants without imposing excessive energy demands or generating additional waste. Conventional methods, such as filtration, often fall short due to their reliance on membranes that can clog and require frequent replacement. As researchers seek more efficient purification technologies, they have been exploring the potential of natural materials, particularly those derived from plants.
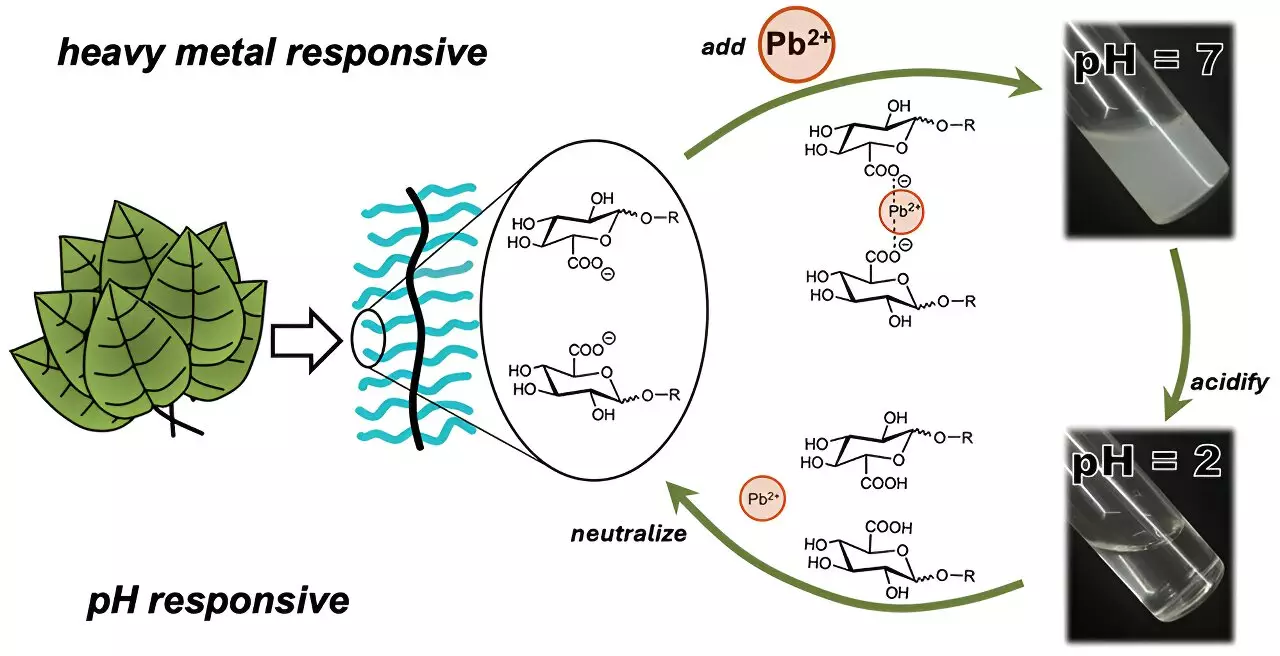
In recent years, the spotlight has turned toward plant-derived polysaccharides, which serve as a natural defense mechanism against threats such as heavy metal ions. These complex carbohydrates form macromolecular structures with repetitive sugar units, allowing them to bind with harmful metals within water. Their application in water purification efforts is compelling—researchers have previously demonstrated success in utilizing polysaccharides from sources like okra and aloe to capture microplastics in wastewater. However, a significant limitation has been the inherent solubility of some polysaccharides. When dissolved, they require additional chemical agents to facilitate the formation of insoluble gels that can capture and remove heavy metals effectively.
Recognizing the need for a more efficient solution, a team of researchers led by Cassandra Callmann at the University of Texas at Austin embarked on an ambitious study to create a novel polymer. This polymer was designed to possess sugar-like structures that would offer adjustable solubility in water, ultimately enhancing its heavy metal trapping capabilities. The researchers synthesized several variants of the polymer, each featuring an insoluble backbone with various water-soluble carbohydrate “charms” that could attract and bind heavy metals.
In their experiments, one particular carbohydrate charm, containing a carboxylic acid group, stood out for its strong affinity for ionic cadmium ions. After introducing this polymer to water deliberately contaminated with cadmium, the results were promising. Within just three minutes, the polymer aggregated into visible clumps, which facilitated easy filtration and removal from the solution.
What sets this polymer apart is its ability to redissolve upon adjusting the water’s acidity, releasing the trapped cadmium for potential reuse while maintaining its metal-trapping efficacy. In a series of tests, the polymer demonstrated remarkable stability, maintaining its metal-binding performance across three cycles of binding, clumping, and redissolving. This feature positions it not only as an efficient water purification barrier but also as a sustainable solution for ongoing water treatment efforts.
Following initial trials, the researchers extended their proof-of-concept by testing this innovative polymer in real-world conditions. They introduced the polymer to samples from the Colorado River, spiking it with ionic cadmium and lead. Remarkably, the polymer was able to capture approximately 20% of the added cadmium and an impressive 45% of the lead over a 24-hour period, while minimizing interactions with other prevalent metal ions, such as calcium, sodium, and magnesium, in the water.
The implications of this research are far-reaching. With the advent of such a versatile polymer, the road to more selective, reusable, and efficient materials for water purification is becoming clearer. By tapping into the innate properties of plant-derived polysaccharides, researchers are paving the way for groundbreaking methods that could transform how wastewater is treated and drinking water is sourced. As society continues to grapple with pollution and environmental degradation, innovations like this polymer signify essential steps toward a more sustainable future—one where clean, safe water is accessible to all.
The ongoing work in this field will undoubtedly inspire further inquiries and technological advancements, combining nature’s ingenuity with human ingenuity to tackle one of the most significant challenges of our time.