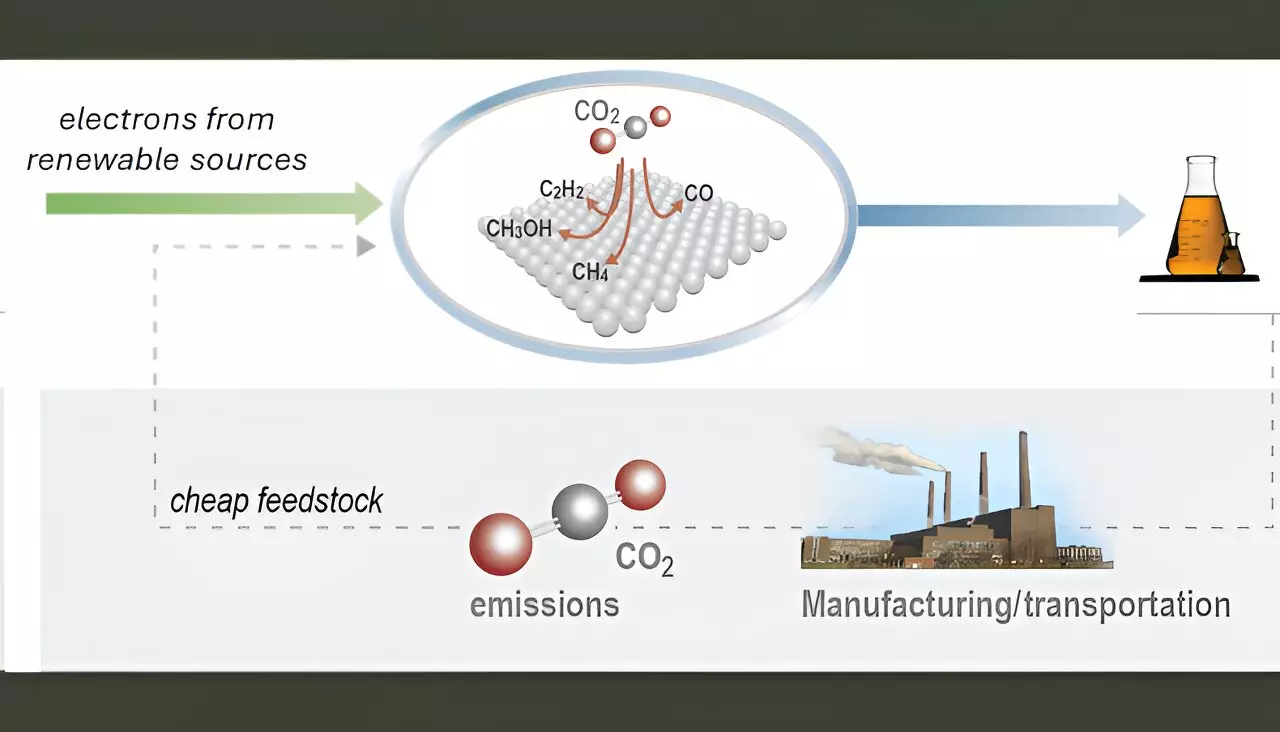
As global focus shifts towards sustainable energy solutions, the coupling of electrochemical conversion of carbon dioxide (CO2) with renewable energy sources, such as solar and wind power, emerges as a beacon of hope for green chemistry. This innovative approach holds the potential to generate high-demand chemicals and fuel, which are critical in mitigating the environmental impacts tied to fossil fuel consumption. Products derived from carbon dioxide, including ethylene, ethanol, and acetic acid, serve as vital feedstocks in the chemical manufacturing sector, providing a pathway to reduce dependency on non-renewable resources.
The integration of renewable electricity into the CO2 conversion process could fundamentally transform the landscape of chemical production and fuel generation. By implementing a method that effectively captures CO2 and utilizes it for creating valuable compounds, industries could not only decrease greenhouse gas emissions but also move towards a circular economy. However, significant hurdles remain before the full commercial viability of these technologies can be realized.
At the heart of the electrochemical conversion process are the catalysts that facilitate the reactions necessary to transform CO2 into useful products. Despite recent advancements in the design of electrolyzers—devices that harness electricity to drive chemical transformations—commercialization has been impeded by concerns surrounding the stability and selectivity of the catalysts. Current research indicates that while various materials can catalyze these reactions, copper and its alloys are the only ones successfully demonstrated to convert CO2 effectively into multi-carbon products.
One prominent obstacle in catalyst development lies in the challenge of isolating catalyst performance from the complications associated with their integration into the electrolyzer systems. With numerous fabrication techniques and varying electrolyzer configurations in use, drawing meaningful comparisons becomes complex and often misleading.
To address the aforementioned challenges, scientists from Lawrence Livermore National Laboratory (LLNL) have forged a pathway towards progress through a novel catalyst coating platform utilizing physical vapor deposition (PVD). This method grants researchers unprecedented control over various catalyst attributes, including thickness, composition, morphology, and porosity. The ability to fine-tune these factors without impacting their integration simplifies the previously complicated task of optimizing catalyst performance.
According to Juergen Biener, a materials scientist at LLNL and a principal contributor to the recent findings, this new scalable and tunable catalyst platform opens the door to enhanced experimental freedom. This flexibility allows for systematic exploration and optimization of copper-based dilute alloy catalysts that have previously been challenging to fabricate and test effectively.
Working collaboratively with researchers from multiple institutions, including the University of Delaware and Washington University, the LLNL team has made significant strides in promoting CO2 conversion efficiencies. By leveraging theoretical frameworks and experimental validation, they created various copper-based dilute alloy catalysts aimed at enhancing the electrochemical reduction pathway. The outcome is a set of catalysts that showcase improved performance characteristics, providing hope for cleaner processes within the chemical and transportation industries.
The implications of this advancement extend beyond mere enhancements in catalyst efficiency. Utilizing PVD techniques not only improves uniformity and control but also reduces waste production, positioning it as a more cost-effective alternative to traditional electrodeposition methods. While initial capital investments may be higher, the long-term benefits could lead to substantial reductions in production costs while fostering environmental sustainability.
As researchers continue to unravel the complexities of CO2 electrolysis, the development of more effective catalysts could invigorate industries reliant on chemical feedstocks and transportation fuels. This progress represents a pivotal shift toward efficiency and sustainability, aligning closely with global goals for carbon neutrality and energy transition.
Ultimately, by revolutionizing the approach to catalyst development, the research being conducted at LLNL and its partners is not just creating new technologies; it is paving the way toward a sustainable future where waste is minimized, resources are responsibly used, and emissions are significantly reduced. The crossroads of innovative catalyst design and renewable energy utilization offers a clear pathway toward achieving lasting environmental change.