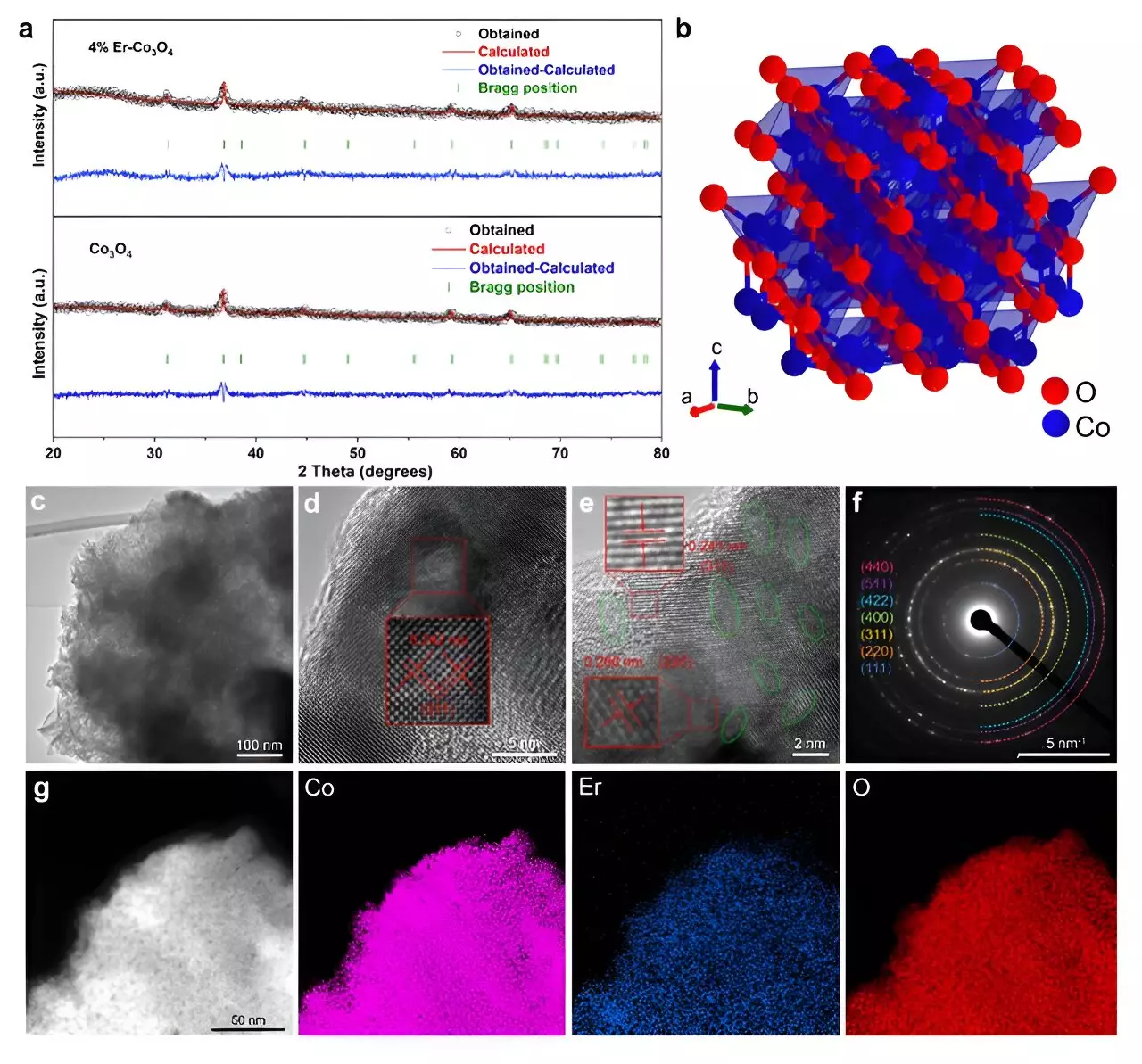
The ongoing quest for efficient and sustainable energy conversion technologies has underscored the importance of oxygen evolution reactions (OER), particularly in the context of green hydrogen production through water splitting. Recent research has unveiled a groundbreaking electrocatalyst that could reshape the energy landscape. A multidisciplinary team has successfully integrated erbium (Er), a rare earth element, into cobalt oxide (Co3O4). This novel approach claims to enhance both the efficiency and stability of OER in acidic environments, charting a path away from the reliance on precious metals that have traditionally dominated this field.
Published in the esteemed journal ACS Catalysis, the study highlights an innovative method that significantly elevates the performance of cobalt-based catalysts. While cobalt oxides have often been lauded for their spinel structure that offers a decent balance between stability and activity, the incorporation of a small quantity of erbium has taken these properties to the next level. According to Tianyi Wang, a leading researcher from Tohoku University’s Advanced Institute for Materials Research, the introduction of a mere 4% erbium into the Co3O4 matrix resulted in a remarkable reduction in overpotential to merely 321 mV at 10 mA cm². More impressively, this newly developed catalyst maintained stability for over 250 hours, rivaling – and in some cases, surpassing – costly iridium-based alternatives.
To dissect the underlying reasons for the enhanced performance, the research team employed sophisticated techniques such as microkinetic modeling and density functional theory (DFT) calculations. Their findings revealed that erbium doping fosters the formation of additional active sites and structural defects within the cobalt oxide framework. This modification increases the ratio of Co3+ to Co2+ ions, which is pivotal for generating oxygen vacancies essential to expedite the OER process.
Hao Li, another prominent co-author, employed a relatable analogy, likening the active sites to additional lanes on a road. Just as extra lanes facilitate traffic flow, the doped oxygen vacancies in the catalyst enhance the movement of necessary intermediates during the reaction. This enhanced traffic flow ultimately translates to increased reaction rates, providing persuasive evidence for the effectiveness of erbium doping.
In situ Raman spectroscopy provided further insight into this phenomenon, revealing that the oxygen vacancies originated in the octahedral sites of the Co3O4 crystal structure. The creation of these vacancies plays a critical role in fostering the development of intermediate species, which are fundamental to the OER mechanism. This study highlights that the optimization of both electronic and structural properties of the catalyst is essential to achieving high performance.
Wang points out that this dual optimization is what differentiates their work from existing catalysts. The balance achieved between structural integrity and electronic functionality creates a highly efficient platform for OER, enabling sustained energy conversion under acidic conditions—a challenge that has persisted in the field of electrocatalysis.
The implications of this research are considerable, not only for the scientific community but also for real-world applications. By utilizing a cost-effective and readily available material like erbium, the researchers envision the potential for developing a new category of electrocatalysts that significantly lower costs without compromising on efficiency or stability.
As the team looks ahead, their focus will expand to explore other non-precious metal doping strategies, aimed at continuously innovating catalytic designs that satisfy the growing energy demands of the future. By pushing the boundaries of what is possible with cost-effective materials, the work of this research team not only paves the way for enhanced energy technologies but also embodies the principles of sustainability and resource responsibility.
The integration of erbium into cobalt oxide catalysts marks a pivotal moment in the realm of electrocatalysis. This innovative research demonstrates that significant strides toward efficiency and stability can be made without recourse to precious metals, reinforcing the importance of continued exploration into alternative materials. The findings underscore a transformative shift in how we approach energy conversion and catalysis, guiding future efforts toward creating a sustainable energy framework that benefits both industry and the environment alike.