In the realm of chemistry, the reliance on sustainable resources is becoming increasingly critical. Dinitrogen (N₂), which constitutes around 78% of the Earth’s atmosphere, stands out as an underutilized raw material. While its abundance is indisputable, the challenge lies in its molecular structure. The strong triple bond between nitrogen atoms makes it resistant to participation in chemical reactions—a hurdle that chemists have long sought to overcome. Recent advancements in this area, particularly from researchers at RIKEN Institute, have illuminated a pathway for using dinitrogen directly in the synthesis of vital compounds, including pharmaceuticals and polymers, potentially revolutionizing industrial chemical processes.
The Current Limitations of Dinitrogen Utilization
The traditional method for utilizing dinitrogen in chemical synthesis involves the Haber-Bosch process, where nitrogen is first converted into ammonia before further transformations can occur. This multi-step procedure not only adds complexity but also consumes a significant amount of energy, contributing to the environmental impact of chemical production. Moreover, the initial requirement to activate alkenes—which are also necessary for creating various nitrogen-containing compounds through alkyl amine synthesis—further complicates the process. Takanori Shima and his colleagues have identified the pressing need for more streamlined alternatives that would allow for a more direct synthesis process, leveraging dinitrogen and alkenes under milder conditions.
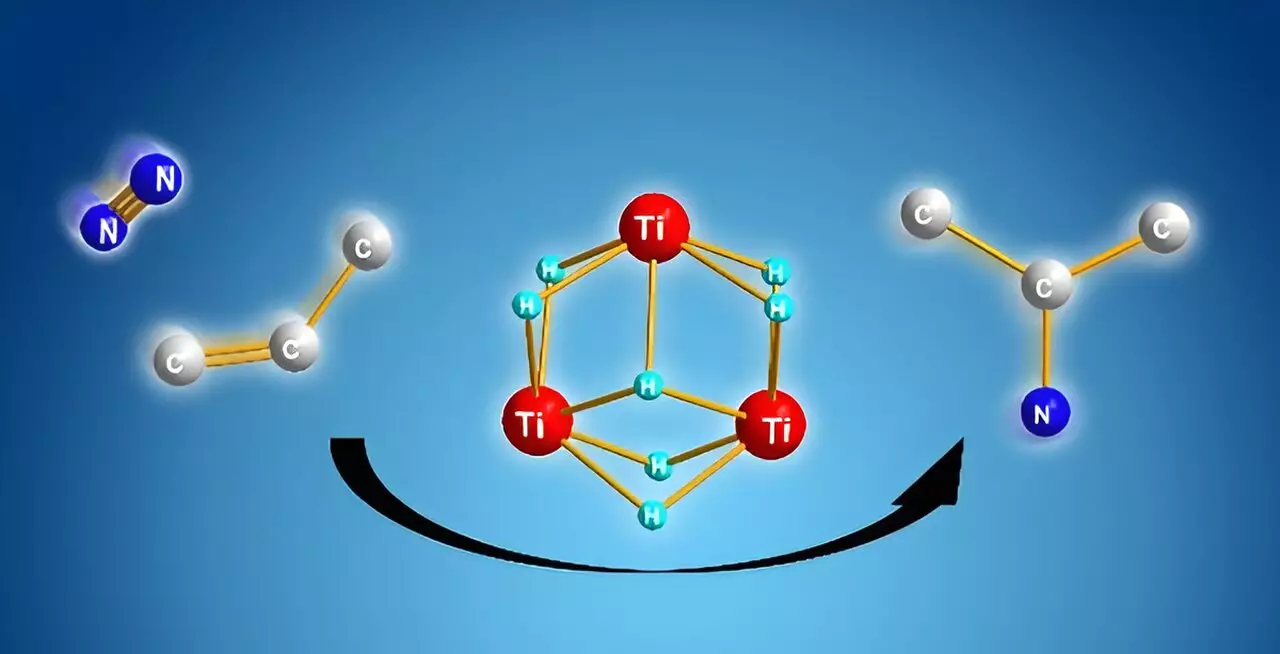
A fundamental breakthrough reported by Shima’s team revolves around the use of titanium polyhydrides, unique complexes where titanium atoms are interconnected by hydrogen atoms. Their research demonstrated that these complexes exhibit remarkable reactivity towards inert molecules such as dinitrogen and benzene. By utilizing the cooperative behavior of the multiple titanium-hydride units, the researchers have enabled a direct reaction pathway for synthesizing alkyl amines from dinitrogen and alkenes. This represents a significant leap forward, as it bypasses the cumbersome pre-activation steps associated with traditional methods.
During experimentation, Shima’s team observed that when alkenes are reacted with titanium polyhydride, the alkenes are activated, while many titanium-hydride units remain available. The introduction of dinitrogen then allows for the cleavage of the nitrogen molecule followed by the formation of new nitrogen-carbon bonds—resulting in the efficient synthesis of alkyl amines. This mechanism reveals the chemoselectivity at play, where the formation of nitrogen-carbon bonds is energetically favored compared to alternative reactions, thereby ensuring a more effective process.
The revelations from this study open up numerous avenues for future exploration. The ability to utilize dinitrogen directly in chemical synthesis not only aligns with the principles of green chemistry but also promises to reduce the overall energy consumption associated with these processes. By integrating a more direct approach to the synthesis of valuable compounds, the potential for industrial application is vast. Shima and his team are currently focusing on the next phase of their research: transforming this one-time reaction into a catalytic process that would allow for its repeated use. Such innovation could significantly lower the cost and energy requirements of producing a wide range of nitrogen-containing compounds.
As the demand for sustainable solutions grows, advancements in chemical synthesis through innovative methodologies like those demonstrated using titanium polyhydrides signify a potential paradigm shift. By unlocking the capabilities of dinitrogen—a molecule that is all around us—scientists can pave the way for more efficient, environmentally friendly chemical production. This breakthrough not only addresses existing challenges in the synthesis of nitrogen and carbon sources but also encourages ongoing research towards developing a more sustainable industrial landscape. The implications of this work are profound, with the power to redefine how essential compounds are synthesized in the future, ultimately contributing to a healthier planet.