Recent advancements in electrochemical methods are paving the way for cleaner and more energy-efficient chemical production. Researchers at Lawrence Livermore National Laboratory (LLNL) have made significant strides in this domain by employing thin film nickel anodes. This innovative approach offers a fresh perspective on how to streamline chemical manufacturing, promising a greener alternative to traditional methods that heavily rely on fossil fuels and emit harmful byproducts.
The utilization of thin films in electrochemical reactions stands out as a crucial innovation. Unlike rough or porous structures that can complicate reactions, thin films provide a uniform and stable surface. This stability is essential for researchers trying to dissect and understand the intricate dynamics at play within catalysts. As LLNL postdoc Aditya Prajapati points out, “Thin films eliminate complications from porous/rough structures or varying thicknesses.” This clear focus facilitates a deeper dive into catalyst performance, ultimately enhancing the research surrounding chemical reactions.
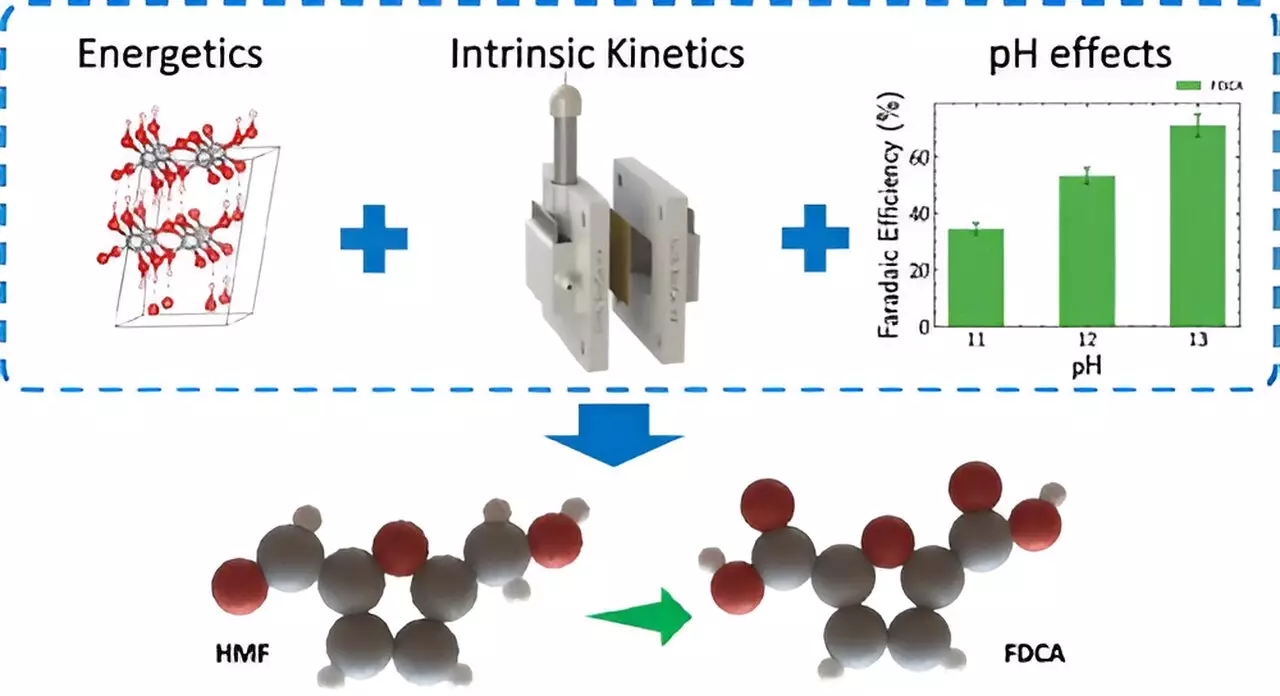
One of the longstanding obstacles in the current electrolyzer technology is the energy inefficiency associated with the oxygen evolution reaction occurring at the anode. The LLNL team’s innovative approach substitutes this reaction with biomass oxidation, resulting in a dramatic reduction in energy consumption—over 50%. By turning to biomass as a resource, the researchers are tackling two critical issues simultaneously: ensuring energy-efficient chemical processes and promoting sustainable biomass conversion.
A key focus of this research is the conversion of 5-Hydroxymethylfurfural (HMF), a biomass-derived compound, into 2,5-Furandicarboxylic acid (FDCA). This transformation yields a crucial building block for producing sustainable plastics, specifically PEF, a bio-based polymer. This process not only diminishes the reliance on petroleum but also marks a significant step towards reducing carbon emissions. As Nitish Govindarajan notes, “Unlike traditional methods that require high temperatures and produce toxic waste, our electrochemical method is cleaner and more energy-efficient.”
The implications of this research extend beyond mere efficiency; it supports a broader vision of a circular economy where renewable resources are prioritized. By leveraging biomass as a raw material, this process emphasizes the importance of sustainable feedstocks while simultaneously curbing the dependence on limited fossil fuel reserves. Additionally, the electrochemical oxidation of HMF to FDCA is inherently less energy-intensive compared to conventional methods, resulting in a smaller carbon footprint for chemical production overall.
The potential for achieving a zero-carbon footprint is one of the most exciting prospects associated with this new electrochemical method. When paired with renewable energy sources, the process holds promise for an eco-friendly revolution in chemistry that not only minimizes environmental impact but also encourages the sustainable utilization of energy. As the field evolves, the collaborative efforts of LLNL researchers and their peers from institutions such as Université de Montréal and the University of Bonn will play a vital role in realizing the vision of cleaner chemical production ecosystems.
This comprehensive approach to improving chemical production reflects a growing awareness of environmental concerns and the need for innovation in sustainability. As expertise in electrochemistry continues to develop, the potential to reshape the industry towards a more sustainable future becomes increasingly achievable.