In the realm of chemistry, the interaction between ions and solvent molecules is critical to understanding numerous physiological and chemical processes. A pivotal concept in this interaction is the solvation shell, which comprises a cluster of solvent molecules that encircle dissolved ions. This layer of solvent is not just a passive entity; rather, the molecules within these shells exhibit unique properties that can significantly differ from free solvent molecules. These differences in properties can influence reaction rates, solubility, and the overall behavior of substances in solution, making solvation shells a focal area of research across various scientific fields, such as biochemistry, materials science, and even environmental science.
Despite the importance of solvation shells, studying their properties has posed considerable challenges for researchers. Traditional methods have struggled to isolate and analyze these complex structures due to the overwhelming presence of other solvent molecules. The inherent dynamic nature of these environments further complicates the ability to obtain meaningful data. Researchers have long sought reliable methods to unravel the behaviors and characteristics of solvation shells, as improvements in this area could augment our understanding of processes ranging from ion transport in biological systems to the development of new materials.
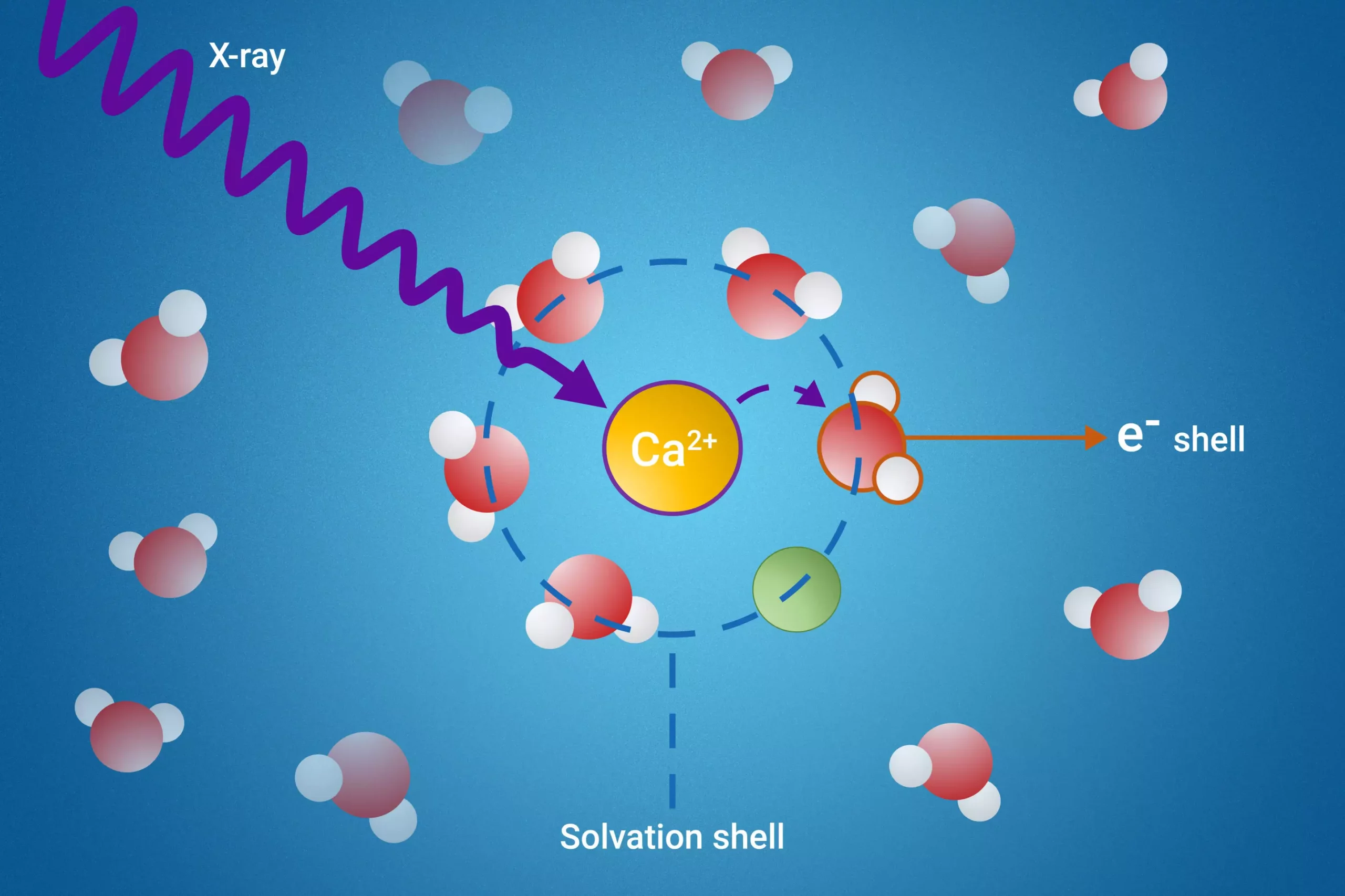
In a groundbreaking study published in *Nature Communications*, a collaborative team of scientists from the Fritz Haber Institute, Sorbonne University, and Uppsala University has introduced a novel method to explore these elusive solvation shells. By employing resonant intermolecular Coulombic decay (ICD), the team can excite molecules with high-energy X-rays and closely monitor their interactions with neighboring molecules during the decay process. This innovative approach allows researchers to obtain detailed insights into the properties of the solvation shells surrounding ions, something that had previously been little understood.
One of the transformative findings of this research is how a particular ICD mechanism serves as a reliable indicator for ion pair formation. The ability to measure electron binding energies of water molecules within the first solvation shell stands out as a significant advancement, particularly since such measurements were previously unachievable. This breakthrough not only propels our understanding of fundamental chemical interactions but also has far-reaching implications for multiple disciplines. For instance, insights gained from this research can inform the development of more effective pharmaceuticals, enhance battery performance, and contribute to more robust environmental models.
This pioneering work greatly enriches the toolkit available to scientists studying solvation shells. As various industries and scientific fields continually seek to push the boundaries of innovation, the ability to probe and understand solvation shells will be crucial. The implications of this research extend well beyond academic interest; it stands to impact practical applications in chemistry, biology, and material sciences. As we continue to unveil the complexities of solvation dynamics, our grasp of molecular behavior in solutions is poised for a significant transformation.