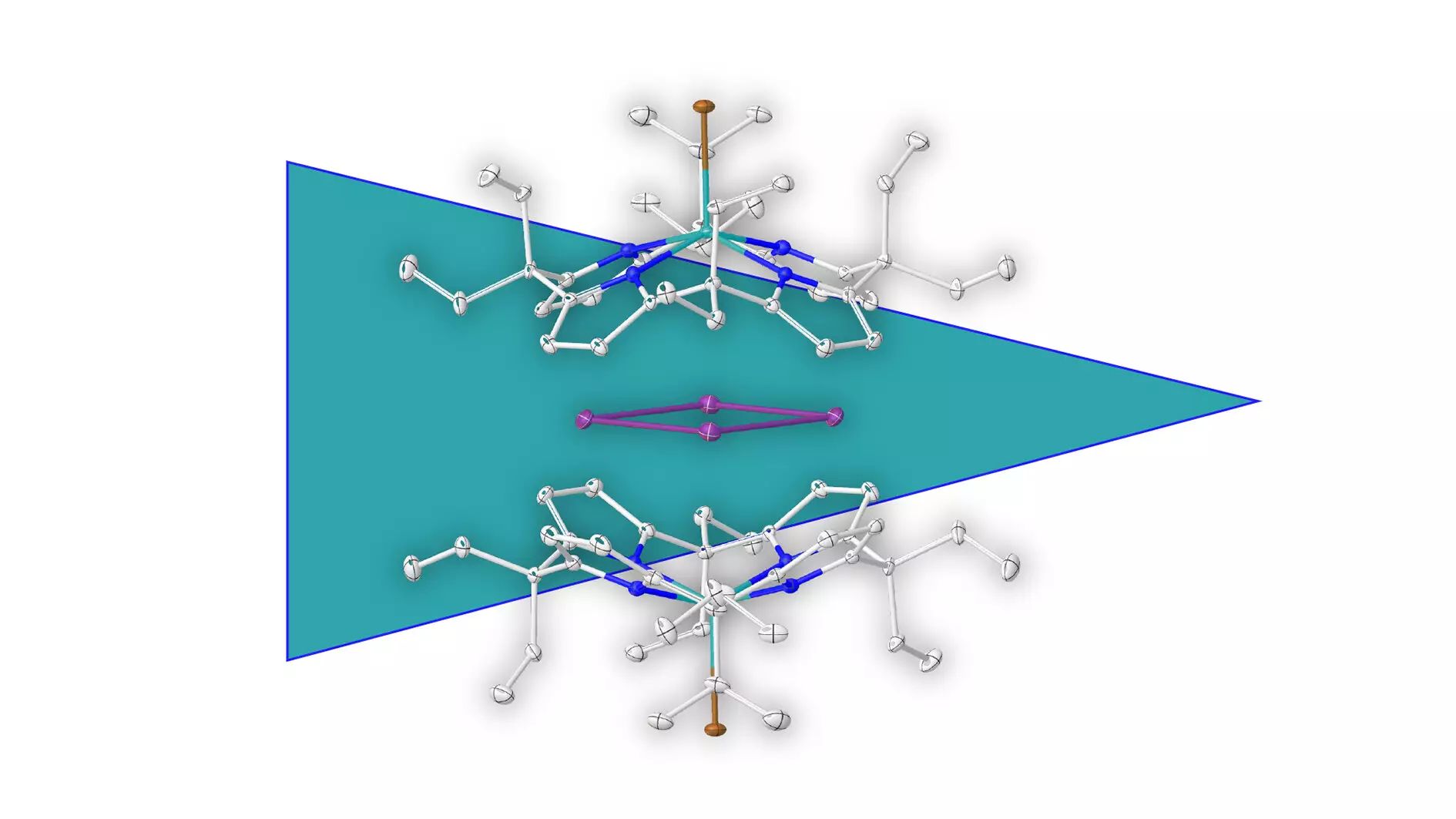
Aromaticity is a foundational concept in the field of chemistry, particularly in the study of organic compounds. Traditionally, aromatic compounds are defined by their ring structures composed primarily of carbon atoms, offering unique stability and reactivity due to their delocalized pi-electron clouds. However, recent research led by Prof. Dr. Lutz Greb at Heidelberg University has ventured into uncharted territory by isolating an aromatic ring formed entirely of metal atoms—specifically, bismuth. This groundbreaking discovery not only challenges long-held beliefs about aromaticity but also opens doors to new possibilities in chemical synthesis and materials science.
The Unexplored Territory of Metal Aromatics
Historically, the predominant focus of aromatic studies has been on organic compounds. The implication that metal rings could exhibit aromatic behavior raises provocative questions about the underlying principles of chemical bonding and stability. The bismuth ring isolated by Greb and his team marks a significant leap forward in our understanding of metal complexes. Unlike previous instances where metal atoms bound to organic aromatic molecules existed, this new paradigm consists of a structure purely formed from metallic elements, suggesting that the essence of aromaticity is not limited to carbon.
Importantly, the research not only highlights a novel compound but also provides insights into the stabilization methods needed to isolate such structures. The use of supramolecular stabilization techniques, wherein a negatively charged molecular shell envelops the positively charged metal ring, showcases innovative strategies that could be applicable in various areas of chemistry. This work suggests that we may be able to stabilize other complex and reactive structures that were previously deemed too unstable for investigation.
Implications for Future Research
The ramifications of isolating a purely metallic aromatic ring extend far beyond a mere curiosity of academic interest. For one, it may significantly impact our understanding of charge transport in metals. Given the increasing relevance of conductive materials in technology and their applications in electronics, the characteristics of this bismuth aromatic compound could lead to advancements in developing more efficient conducting materials. Greb’s assertion that this discovery could spark a foundational shift in our comprehension of aromaticity propels the conversation toward a future where the boundaries of chemical classifications blur.
Furthermore, the presence of aromaticity in metal-only structures may inspire a more profound investigation into the roles of other elemental categories in aromatic behavior. This hints at a potential reevaluation of established theories in chemistry, inviting scientists to examine how varied configurations of elemental arrangements influence stability and reactivity.
The research led by Prof. Dr. Lutz Greb not only breaks new ground but also paves the way for innovations in chemical science and material engineering. With a shift in perspective regarding aromatic structures, the potential for future discoveries remains exhilarating. The isolation and characterization of the bismuth ring could usher in an era where metallic aromatic compounds play a pivotal role in the evolution of various applications, reinforcing the idea that chemistry’s most exciting discoveries lie at the intersection of exploration and innovation.