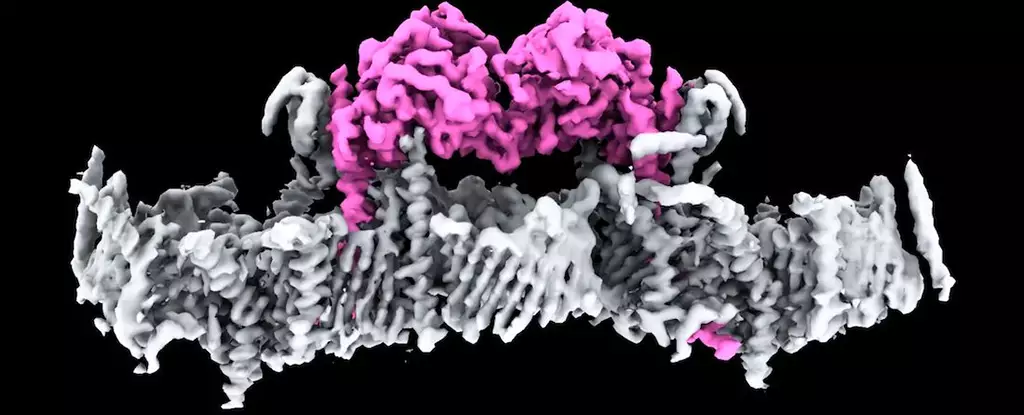
For the first time, the intricate dance of mitochondrial health and its impact on Parkinson’s disease has come under the microscope thanks to groundbreaking research from the Walter and Eliza Hall Institute of Medical Research (WEHI) in Australia. The team has unraveled the structure of the PINK1 protein, a component critical to maintaining cellular energy levels. This discovery marks a watershed moment in understanding how mutations in the PINK1 gene can lead to early-onset Parkinson’s disease, a condition that has perplexed scientists for decades.
The PINK1 protein has long been recognized as a key player in mitochondrial maintenance, acting as a guardian of energy-producing structures within the cell. It does this with pinpoint precision, passing through mitochondrial membranes to perform its duties. The research confirms that in healthy cells, PINK1 operates flawlessly, whereas in dysfunctional mitochondria, it fails to fulfill its role, leading to catastrophic cellular consequences. This newly obtained insight brings us closer to understanding the disease’s biochemical underpinnings, opening doors for potential therapeutic interventions.
Advanced Imaging Techniques Pave the Way
The pioneering techniques of cryo-electron microscopy and mass spectrometry used in this research are remarkable in their capacity to reveal details at the molecular level. By employing these advanced methods, the researchers identified how PINK1 interacts with mitochondria and, crucially, identified a specific protein complex known as TOM-VDAC critical for this interaction. This precise docking mechanism, coupled with the insight on the consequences of genetic mutations in PINK1, provides a comprehensive picture of the events leading to neurodegeneration.
David Komander, a medical biologist at WEHI, succinctly captures the significance of this research. By elucidating how PINK1 binds to mitochondria, the team has unlocked new avenues for therapeutic strategies that could one day switch on the PINK1 protein effectively. These findings not only highlight the protein’s role but also illuminate the broader mechanisms at play in mitochondrial dysfunction related to Parkinson’s disease.
The Consequences of Dysfunction
Understanding the ramifications of PINK1 malfunctions is paramount in the discourse surrounding Parkinson’s disease. When PINK1 is mutated, not only does it disrupt its normal activity, but it also fails to mark damaged mitochondria for removal. This dysfunction leads to an accumulation of impaired cellular powerhouses, creating an energy deficit that ultimately affects brain health. Given that neuronal cells are among the most energy-demanding cells in the body, an inefficient replacement system for mitochondria could spell disaster, paving the way for neurodegeneration associated with Parkinson’s.
Moreover, the research underscores the importance of mitochondrial health beyond Parkinson’s, positioning PINK1 as a crucial protein in the broader landscape of neurodegenerative diseases. As scientists continue to unravel the complexities of such mechanisms, they may find that the pathways affected by PINK1 are shared with other neurological conditions, amplifying its significance in neurology.
Looking to the Future
While the research is still in its nascent stages, the promise of developing treatments aimed at repairing PINK1 functionality offers a glimmer of hope to those afflicted by Parkinson’s disease. If successful strategies can be identified that effectively restore PINK1’s ability to clear dysfunctional mitochondria, the therapies could represent a monumental shift in how we manage not just Parkinson’s but potentially other related neurological disorders.
Sylvie Callegari, a biochemist with WEHI, enriches the narrative by emphasizing the first-time visualization of human PINK1 bound to damaged mitochondria, noting the remarkable array of docking proteins identified in the process. Each of these findings contributes to a mosaic of knowledge that researchers can leverage as they investigate the multifaceted nature of Parkinson’s disease.
As the team continues its investigations, the interconnectedness of genetics, protein functionality, and mitochondrial health becomes increasingly apparent. Armed with this new knowledge, the scientific community stands on the cusp of potentially transformative advancements that could redefine how we approach neurodegenerative diseases. By focusing on the minutiae of cellular functions and their failures, researchers are not just dissecting the protein; they are laboring towards uncovering life-altering solutions for millions.