The escalating challenge of antimicrobial resistance (AMR) presents a formidable obstacle for healthcare systems globally. This resistance not only complicates the treatment of infections but also raises alarm bells among public health officials. As bacteria evolve and outsmart existing medications, it is crucial that researchers innovate relentlessly to develop effective alternatives. At the forefront of this critical endeavor is a team from Hokkaido University, led by Assistant Professor Kazuki Yamamoto and Professor Satoshi Ichikawa, who are crafting a novel approach that could change the landscape of antibiotic discovery.
Innovative Strategies in Drug Discovery
Yamamoto and Ichikawa’s groundbreaking research, published in the esteemed journal Nature Communications, reveals an impressive methodology aimed at streamlining the identification of new antimicrobial agents. Central to their strategy is the enzyme phospho-N-acetylmuramoyl-pentapeptide-transferase (MraY), a pivotal player in bacterial cell wall synthesis. MraY catalyzes the assembly of lipid I, an essential component that fortifies bacterial life. Their work illustrates an urgent need for enhanced inhibitors of MraY, as existing options fall short in combatting the relentless grip of AMR.
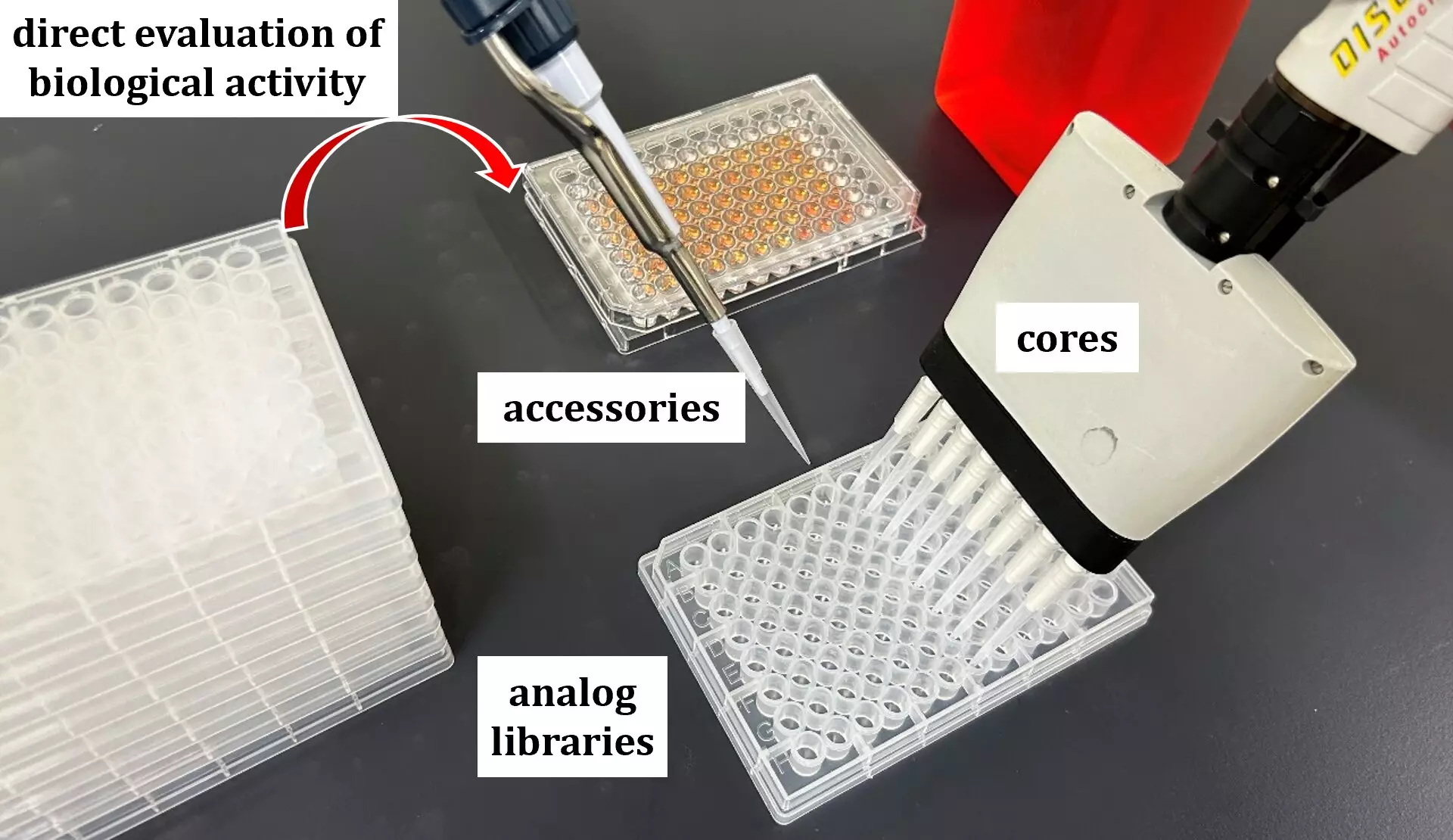
The researchers adopted a cutting-edge framework they dubbed the “in situ build-up library method.” This technique melds comprehensive synthesis of natural product derivatives with direct biological activity evaluations, bridging the gap between theoretical chemistry and practical pharmacology. By disassembling known MraY inhibitors into their foundational components—cores and accessory groups—Yamamoto’s team crafted a library of 686 analogs, tailored for rigorous testing against MraY.
Transforming Science into Therapeutics
The ingenuity behind their approach lies in the simplicity and efficacy of the methodology. By attaching reactive groups to the cores and accessories of the MraY inhibitors, the team catalyzed the formation of a hydrazone bond, facilitating the creation of a diverse library of analogs. Among these, eight candidates exhibited robust MraY inhibition and antibacterial properties, with analog 2 shining the brightest. This particular analog’s exceptional performance against drug-resistant strains, coupled with promising results in mouse models, marked a significant milestone in the transition from laboratory discovery to clinical application.
The ability to demonstrate efficacy in living organisms establishes a critical precedent for these novel candidates. This step not only validates their therapeutic potential but also underscores the importance of safety profiles; early findings indicate that these compounds show low toxicity to non-target human cells—a promising signal for future development.
Broadening Horizons: Beyond Antibiotics
Yamamoto and Ichikawa’s research extends beyond antibiotics, revealing a versatile framework for drug discovery that can be adapted to various therapeutic classes. With their method proving effective in deriving analogs from tubulin-binding natural products such as epothilone B and paclitaxel—well-known anti-cancer agents—the implications of their work resonate across multiple fields of drug development. In just one month, the researchers constructed a library of 588 analogs, exemplifying not only the efficiency of their methodology but also its potential breadth.
This pioneering research heralds a new chapter in the war against drug-resistant pathogens. As AMR continues to challenge health professionals, the ability to create a library of promising drug candidates quickly and effectively could spell the difference between life and death for countless patients.
A Future with Renewed Optimism
While the fight against AMR remains daunting, the novel insights and methodologies emerging from Hokkaido University signify a growing reservoir of hope. Continuous advancements in drug development techniques not only promise a more rapid identification of effective treatments but also shine a light on interdisciplinary approaches that can revolutionize the pharmaceutical landscape. As researchers tackle the pressing need for effective antimicrobials with renewed vigor, the potential for innovative solutions to flourish becomes clearer. The journey toward a sustainable and resilient arsenal of antibiotics is fraught with challenges, yet the dedication and creativity of modern science prevail.